CBSE 12th exams are underway and your CBSE 10th Chemistry exam is scheduled on 27th Feb, 2025. You have just a few hours left for CBSE 12th Chemistry exam.
We have prepared Top 50 MCQs with answers covering all the important topics of CBSE Class 12 Chemistry syllabus.
Make sure to solve these MCQs and refer to the solutions to ensure that you are well prepared to score high in CBSE Class 12 Chemistry exam 2025.
👉 Read Also - CBSE Class 12 Chemistry 2025: Chapter-Wise Competency-Based Questions with Solutions – Free PDF Download
CBSE 12th Chemistry 2025: Top 50 MCQs with Answers
1. Osmotic pressure of a solution is 0.0821 atm at a temperature of 300 K. The concentration in moles/litre will be:
(a) 0.33
(b) 0.666
(c) 0.3 × 10-2
(d) 3
Answer ⇒ (c) 0.3 × 10-2
2. The boiling point of an azeotropic mixture of water and ethanol is less than that of water and ethanol. The mixture shows:
(a) no deviation from Raoult's Law.
(b) positive deviation from Raoult's Law.
(c) negative deviation from Raoult's Law.
(d) that the solution is unsaturated.
Answer ⇒ (b) positive deviation from Raoult's Law.
3. Isotonic solutions have the same (CBSE 2024)
(a) density
(b) refractive index
(c) osmotic pressure
(d) volume
Answer ⇒ (c) osmotic pressure
👉 Read Also - CBSE Class 12 Chemistry Exam 2025: Important Questions, PYQs & Sample Papers for All Chapters - Free PDF Download
4. The van't Hoff factor (i) accounts for:
(a) degree of solubilisation of solute.
(b) the extent of dissociation of solute.
(c) the extent of dissolution of solute.
(d) the degree of decomposition of solution.
Answer ⇒ (b) the extent of dissociation of solute.
5. For determination of molar mass of polymersand proteins, which colligative property is used? (2021)
(a) Relative lowering in vapour pressure
(b) Elevation in boiling point
(c) Osmotic pressure
(d) Depression in freezing point
Answer ⇒ (c) Osmotic pressure is widely used to determine molar masses of proteins, and polymers.
6. NH4NO3 is used in salt bridge because
(a) it forms a jelly-like material with agar-agar.
(b) it is a weak electrolyte.
(c) it is a good conductor of electricity.
(d) the transport number of NH+4 and NO-3 ions are almost equal.
Answer ⇒ (d) the transport number of NH+4 and NO-3 ions are almost equal.
7. Standard electrode potentials of three metals X, Y and Z are -1.2 V, +0.5 V and -3.0 V respectively. The reducing power of these metals will be:
(a) Y > Z > X
(b) Y > X > Z
(c) Z > X > Y
(d) X > Y > Z
Answer ⇒ (c) As the reduction potential decreases, the reducing power of the electrode increases.
∴ Z(-3.0 V) > X(-1.2 V) > Y(+0.5 V)
👉 Read Also - CBSE Board Class 12 Chemistry Exam 2025 : Chapter-Wise Most Predicted Questions with Answers; Download Free PDF
8. Which of the following is correct for spontaneity of a cell? (2020 HMJ/4)
(a) ΔG = -ve, E° = +ve
(b) ΔG = +ve, E° = 0
(c) ΔG = -ve, E° = 0
(d) ΔG = +ve, E° = -ve
Answer ⇒ (a) ΔG = -ve, E° = +ve
9. Kohlrausch has given the following relation for strong electrolytes:
Λ = Λ0 - A√C
Which of the following equality holds? (2020 HMJ/5)
(a) Λ = Λ0 as C → √A
(b) Λ = Λ0 as C → ∞
(c) Λ = Λ0 as C → 0
(d) Λ = Λ0 as C → 1
Answer ⇒ (c) Λ = Λ0 as C → 0
10. The half-life of the first-order reaction having rate constant K = 1.7 × 10⁻⁵ s⁻¹ is:
(a) 12.1 hrs
(b) 9.7 hrs
(c) 11.3 hrs
(d) 1.8 hrs
Answer ⇒ (c) 11.3 hrs
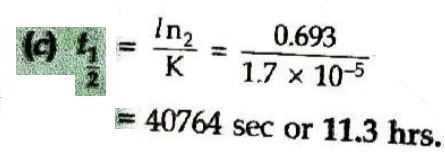
11. CuSO4. 5H2O is blue in colour because
(a) It contains water of crystallization.
(b) SO42- ions absorb red light.
(c) Cu2+ ions absorb orange red light.
(d) Cu2+ ions absorb all colours except red from the white light.
Answer ⇒ (a) It contains water of crystallization.
12. Which one of the following characteristics of the transition metals is associated with higher catalytic activity?
(a) High enthalpy of atomisation
(b) Paramagnetic behaviour
(c) Colour of hydrate ions
(d) Variable oxidation states
Answer ⇒ (d) Variable oxidation states
👉 Read Also - CBSE Class 12 Chemistry Board Exam 2025 : Most Repeated Questions from Last 10 Years; Download PDF
13. The property which is not characteristic of transition metals is
(a) variable oxidation states.
(b) tendency to form complexes.
(c) formation of coloured compounds.
(d) natural radioactivity.
Answer ⇒ (d) natural radioactivity.
14. Transition metals, despite high E° oxidation, are poor reducing agents. The incorrect reason is
(a) high heat of vaporisation.
(b) high ionisation energies.
(c) low heats of hydration.
(d) complex forming nature.
Answer ⇒ (d) complex forming nature.
15. Which of the following characteristics of transition metals is associated with their catalytic activity? (2023 Series: HFG1E/2)
(a) Paramagnetic nature
(b) Colour of hydrated ions
(c) High enthalpy of atomisation
(d) Variable oxidation states
Answer ⇒ (d) Due to formation of variable oxidation states, transition metals show catalytic property.
16. Which property of transition metals enables them to behave as catalysts? (2023 Series: HFG1E/5)
(a) High melting point
(b) High ionisation enthalpy
(c) Alloy formation
(d) Variable oxidation states
Answer ⇒ (d) Due to variable oxidation states transition metals can form complexes which make them a good catalyst.
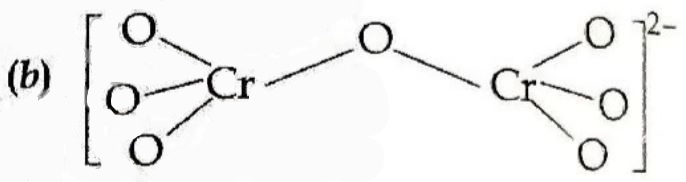
17. In the two tetrahedral structures of dichromate ion. (2023 Series: HFG1E/5)
(a) 4 Cr—O bonds are equivalent in length.
(b) 6 Cr—O bonds are equivalent in length.
(c) All Cr—O bonds are equivalent in length.
(d) All Cr—O bonds are non-equivalent.
Answer ⇒ (b) 6 Cr—O bonds are equivalent in length.
18. The correct order of the first ionisation enthalpies is.
(a) Ti < Mn < Zn < Ni
(b) Mn < Ti < Zn < Ni
(c) Zn < Ni < Mn < Ti
(d) Ti < Mn < Ni < Zn
Answer ⇒ (d) As the size decreases along the period, the ionisation enthalpies increase.
19. Which of the following statement is wrong?
(a) All elements of 3d series exhibit variable oxidation states.
(b) Metals in highest oxidation states are more stable in oxides than in fluorides.
(c) Mn³⁺ and Co³⁺ are oxidising agents in aqueous solution.
(d) In highest oxidation states, the transition metals show acidic character.
Answer ⇒ (a) Sc and Zn do not show variable oxidation states.
20. Identify the incorrect statement from the following:
(a) Lanthanoids reveal only +3 oxidation state.
(b) Zr and Hf have identified radii of 160 pm and 159 pm respectively as a consequence of lanthanoid contraction.
(c) The overall decrease in atomic and ionic radii from lanthanum to lutetium is called lanthanoid contraction.
(d) The lanthanoid ions other than the f⁰ type and the f¹⁴ type are all paramagnetic.
Answer ⇒ (a) Lanthanoids also show variable oxidation state of +3 and +4.
21. The IUPAC name of K₃[Fe(CN)₅NO] is:
(a) tripotassium pentacyanonitrosylferrate (II)
(b) potassium pentacyanonitrosylferrate (III)
(c) potassium pentacyanonitroferrate (II)
(d) potassium pentacyanonitrosylferrate (II)
Answer ⇒ (d) potassium pentacyanonitrosylferrate (II)
22. IUPAC name of [Pt(NH₃)₃ Br(NO₂) Cl] Cl is:
(a) triamminechlorodibromidoplatinum (IV) chloride
(b) triamminechloridobromidonitrochloride-platinum (IV) chloride
(c) triamminebromidochloridonitroplatinum (IV) chloride
(d) triamminenitrochlorobromoplatinum (IV) chloride
Answer ⇒ (c) triamminebromidochloridonitroplatinum (IV) chloride
23. Trunbull’s blue is:
(a) Ferricyanide
(b) Ferrous ferricyanide
(c) Ferrous cyanide
(d) Fe₃[Fe(CN)₆]₄
Answer ⇒ (b) Ferrous ferricyanide
24. Which of the following has square planar structure?
(a) [NiCl₄]²⁻
(b) [Ni(CO)₄]
(c) [Ni(CN)₄]²⁻
(d) None of these
Answer ⇒ (c) [Ni(CN)₄]²⁻
25. Which of the following ligands is an ambidentate ligand? (2023 Series: HFG1E/2)
(a) CO
(b) NO₂
(c) NH₃
(d) H₂O
Answer ⇒ (b) NO₂ can donate electron from either oxygen or nitrogen atom of its molecule and forms O−N−O and −NO₂ linkage.
26. The formula of the complex Iron (III) hexacyanidoferrate (II) is: (2023 Series: HFG1E/2)
(a) Fe₂[Fe(CN)₆]₃
(b) Fe₄[Fe(CN)₆]₃
(c) Fe[Fe(CN)₆]
(d) Fe₃[Fe(CN)₆]₂
Answer ⇒ (b) Fe₄[Fe(CN)₆]₃
27. The pair [Co(NH₃)₄Cl₂]Br₂ and [Co(NH₃)₄Br₂]Cl₂ will show (2020 Series: HMJ/4)
(a) Linkage isomerism
(b) Hydrate isomerism
(c) Ionization isomerism
(d) Coordinate isomerism
Answer ⇒ (c) Ionization isomerism
28. What is the secondary valency of Cobalt in ? (2023 Series: HFG1E/2)
(a) 6
(b) 4
(c) 2
(d) 8
Answer ⇒ (a) 6
Secondary valencies have characteristic spatial arrangements in coordination polyhedra and represent number of donor atoms in ligands.
29. SN1 reaction of alkyl halides leads to
(a) Retention of configuration
(b) Racemisation
(c) Inversion of configuration
(d) None of the above
Answer ⇒ (b) Racemisation
30. p-Dichlorobenzene has higher melting point than its o- and m- isomers because (Competency)
(a) p-Dichlorobenzene is more polar than o- and m-isomer.
(b) p-Isomer has a symmetrical crystalline structure.
(c) Boiling point of p-isomer is more than o- and m-isomer.
(d) All of these are correct reasons.
Answer ⇒ (b) p-Isomer has a symmetrical crystalline structure.
31. Chloropicrin is formed by the reaction of
(a) Steam on carbon tetrachloride.
(b) Nitric acid on chlorobenzene.
(c) Chlorine on picric acid.
(d) Nitric acid on chloroform.
Answer ⇒ (d) Nitric acid on chloroform.
32. Auto-oxidation of chloroform in air and light produces a poisonous gas known as (CBSE 2024)
(a) Phosphine
(b) Mustard gas
(c) Phosgene
(d) Tear gas
Answer ⇒ (c) Phosgene
33. Which of the following alcohols will not undergo oxidation? (2023 Series: HFG1E/5)
(a) Butanol
(b) Butan-2-ol
(c) 2-Methylbutan-2-o1
(d) 3-Methylbutan-2-ol
Answer ⇒ (c) 2-Methylbutan-2-ol is a tertiary alcohol which doesn’t undergo oxidation.
34. The correct order of boiling point of primary (1°), secondary (2°) and tertiary (3°) alcohols is
(a) 1° > 2° > 3°
(b) 3° > 2° > 1°
(c) 2° > 1° > 3°
(d) 2° > 3° > 1°
Answer ⇒ (a) 1° > 2° > 3°
35. Which of the following reagents cannot be used to distinguish between phenol and benzyl alcohol?
(a) FeCl₃
(b) Litmus soln
(c) Br₂/CCl₄
(d) All of the above
Answer ⇒ (c) Br₂/CCl₄
36. Nucleophilic addition of Grignard reagent to ketones followed by hydrolysis with dilute acids forms: (CBSE 2024)
(a) Alkene
(b) Primary alcohol
(c) Tertiary alcohol
(d) Secondary alcohol
Answer ⇒ (c) Tertiary alcohol
37. Which of the following cannot reduce fehling solution?
(a) Formic acid
(b) Acetic acid
(c) Formaldehyde
(d) Acetaldehyde
Answer ⇒ (b) Acetic acid has no aldehyde group and does not reduce fehling solution.
38. Which of the following would not be a good choice for reducing nitrobenzene to aniline? (2023 Series: HFG1E/5)
(a) LiAlH₄
(b) H₂/Ni
(c) Fe and HCl
(d) Sn and HCl
Answer ⇒ (a) LiAlH₄ is not used for reduction of nitrobenzene to aniline as it is dangerous for the reaction.
👉 CBSE Class 12 Study Materials
| CBSE Class 12 Syllabus 2024-25 | CBSE Class 12 Previous Year Papers |
| NCERT Books For Class 12 Books | NCERT Class 12 Solutions |
| CBSE Class 12 Full Study Material | CBSE Class 12 Sample Paper 2024-25 |
39. Primary and secondary amines cannot be distinguished by:
(a) Schiff’s reagent.
(b) Carbylamine reaction.
(c) Hoffmann’s bromamide reaction.
(d) Iodoform test.
Answer ⇒ (b) Carbylamine reaction.
40. The correct decreasing order of boiling points among amines and their corresponding acids and alcohols is:
(a) R–CH2NH2 > RCOOH > RCH2OH
(b) RCH2NH2 > RCH2OH > RCOOH
(c) R–CH2OH > R–CH2NH2 > RCOOH
(d) R–COOH > R–CH2OH > R–CH2NH2
Answer ⇒ (d) R–COOH > R–CH2OH > R–CH2NH2
41. Aniline is less basic than ethylamine. This is due to:
(a) conjugation of lone pair of nitrogen with the ring.
(b) the insoluble nature of aniline.
(c) more Kb value of aniline.
(d) hydrogen bonding.
Answer ⇒ (a) conjugation of lone pair of nitrogen with the ring.
42. Nitration of aniline is carried out after acylation because:
(a) acylation deactivates the –NH₂ group.
(b) oxidation can be prevented.
(c) o- and p-products are obtained in good yield.
(d) All of these
Answer ⇒ (d) All of these
43. The sugar present in milk is
(a) Sucrose
(b) Maltose
(c) Glucose
(d) Lactose
Answer ⇒ (d) Lactose
44. Distinction between glucose and fructose can be done by
(a) Benedict’s solution
(b) Tollen’s reagent
(c) Selivanoff’s reagent
(d) Fehling solution
Answer ⇒ (c) Selivanoff’s reagent
45. Hydrolysis of sucrose gives
(a) Glucose only
(b) Glucose + fructose
(c) Glucose & galactose
(d) Maltose
Answer ⇒ (b) Glucose + fructose
46. The specific sequence in which amino acids are arranged in a protein is called its (CBSE 2024)
(a) Primary structure
(b) Secondary structure
(c) Tertiary structure
(d) Quaternary structure
Answer ⇒ (a) Primary structure
47. DNA and RNA differ in
(a) Sugar
(b) Purines
(c) Pyrimidines
(d) Both (a) and (c)
Answer ⇒ (d) Both (a) and (c)
48. β-pleated sheet structure in proteins refers to (2023 Series: HFG1F/2)
(a) primary structure
(b) secondary structure
(c) tertiary structure
(d) quaternary structure
Answer ⇒ (b) secondary structure
49. Two among the three components of DNA are 2-deoxyribose and a nitrogen-containing heterocyclic base. The third component is (2021)
(a) D-ribose
(b) Thymine
(c) Guanine
(d) Phosphoric acid
Answer ⇒ (d) Phosphoric acid
50. Which one among the following bases is usually not present in RNA? (2021)
(a) Uracil
(b) Thymine
(c) Adenine
(d) Guanine
Answer ⇒ (b) Thymine is present in DNA and binds to adenine.
👉 Download PDF - CBSE Class 12 Chemistry Exam 2025 - Top 50 MCQs with Answers







 Profile
Profile Signout
Signout








 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










