Overview Of The Consortium Of Medical, Engineering, And Dental Colleges Of Karnataka Under Graduate Entrance Test
COMEDK stands for Consortium of Medical, Engineering, and Dental Colleges of Karnataka. With the COMEDK Under Graduate Entrance Test, you can get admission into various fields across different colleges in the state of Karnataka. You can get into BE, B.Tech, and other courses by clearing this exam. For more information, we have made a table below with which you can understand all the important points.
|
Feature |
Description |
|
Examination Name |
COMEDK UGET: Consortium of Medical, Engineering, and Dental Colleges of Karnataka Under Graduate Entrance Test |
|
Exam Conducting Organization |
Consortium of Medical, Engineering, and Dental Colleges of Karnataka |
|
Mode of the exam |
Computer-based test |
|
Courses offered |
BE, B.Tech, and B.Arch and others |
|
Frequency of the exam |
Conducted once a year |
|
Medium of the exam |
English |
|
Name of the subjects included in the exam |
It has three major subjects
|
|
Types of Questions |
Objective type Questions |
|
Total Questions and marks in the CBT |
|
|
Marking scheme of the exam |
|
|
Duration of the exam |
3 hours |
|
Official website |
|
|
Availability of COMEDK Mock Test free |
Subject-Wise COMEDK Mock Test 2026
On our website, you will get the COMEDK Mock Test 2026 divided subjectively according to the syllabus. It contains three subjects which are Physics, Chemistry, and Mathematics. You can read how each subject is important for you and what would you learn by attempting the subject-wise Mock Tests.
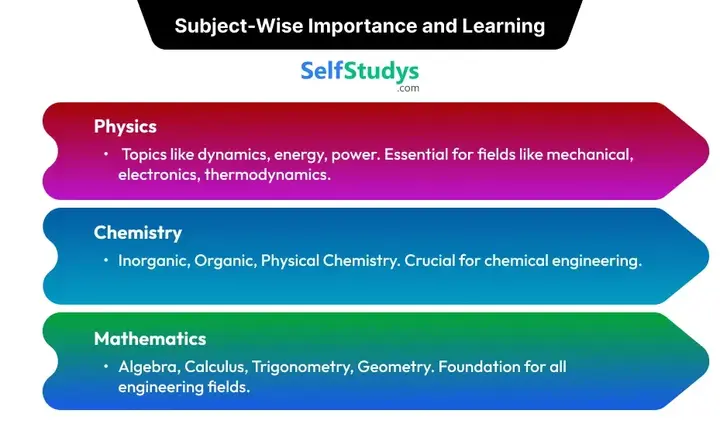
COMEDK Physics Mock Test 2026
It is one of the important subjects for understanding engineering concepts and is most helpful for you if you want to get into the fields of mechanical, electronics, and thermodynamics. In this COMEDK Online Mock Test, you will learn about topics like dynamics, energy, power, and others which will help you to clear your concepts. Repeated practice of the Mock Test will help you in memorizing the formulas more accurately and effectively.
COMEDK Chemistry Mock Test 2026
Chemistry is also important for the chemical engineering field as it will help you understand the properties and interactions of different materials. Inorganic Chemistry, Organic Chemistry, and Physical Chemistry which are available in the COMEDK Mock Test series on our platform should be practiced by you to score good marks. By exercising it regularly, you can excel in balancing equations and solving reactions with in-depth knowledge of the concept. This subject contains both theoretical and practical knowledge, so, by attempting these Questions you can grasp both of them properly and prepare for the real examination.
COMEDK Maths Mock Test 2026
This subject has been taught since the very beginning of schooling. So, this is the backbone subject for all the fields of engineering. It will help you get into civil, mechanical, computer science, and electrical engineering. And, practicing the COMEDK Online Mock Test free for the Maths subject will help you to master all the important concepts like Algebra, Calculus, Trigonometry, and Geometry. You can speed up in solving the Questions and improve your logical reasoning skills as well.
COMEDK Online Mock Test With Solutions
All the Questions given in the COMEDK Mock Test are created along with accurate solutions by highly educated professionals of SelfStudys. These solutions are explained in a step-by-step method with which you can understand each Question easily. You will get the solutions after completing and submitting the test on our digital platform. By reviewing the solutions, you can analyze your right and wrong responses. Make sure you use the given solutions correctly and learn from them to avoid any mistakes in the next Mock Test attempt.
How To Attempt The COMEDK Mock Test Free On SelfStudys?
To attempt the COMEDK Mock Test free on our platform, you have to follow some steps given below carefully.
- The first step you have to do is to open your favourite web browser and type www.selfstudys.com in the search panel.
- When you land on our homepage, you have to tap on the “Navigation” icon displayed on the left side of the screen.

- A new menu will show various study resources and you should click on the “JEE” option.
- This option now showcases a drop-down menu with multiple practice materials where you have to select the “COMEDK Mock Test” icon.
- Then, a new page will load on your screen and you will see all the subjects of the COMEDK Mock Test 2026.
- You can choose any subject to practice the COMEDK Mock Test free Online.
- After choosing the subject from the COMEDK Mock Test series, you can check out all the sets available and select one to solve.
- At last, you just need to tap on the “Start Test” button of the COMEDK Online Mock Test and solve its Questions.
Syllabus Of The COMEDK Under Graduate Entrance Test
Here is a detailed view of the syllabi for all the subjects. You can check the topics mentioned in each unit with the help of the tables below.
Physics
Physics will give you a detailed study of the magnets, electrons, energy, work, and other related topics.
|
Physics XI |
|
|
UNITS |
TOPICS |
|
Physical World and Measurement |
Units and Measurements |
|
Dimensions of physical quantities, dimensional analysis, and its applications. |
|
|
Kinematics |
Motion in a Straight Line Frame of reference, Motion in a straight line: Elementary concepts of differentiation and integration for describing motion Uniform and non-uniform motion, average speed and instantaneous velocity, Uniformly accelerated motion, velocity-time, and position-time graphs. Relations for uniformly accelerated motion (graphical treatment). |
|
Motion in a Plane Scalar and vector quantities; Position and displacement vectors, general vectors and their notations; equality of vectors, multiplication of vectors by a real number; addition and subtraction of vectors. Unit vector; Resolution of a vector in a plane - rectangular components. Scalar and Vector product of vectors. |
|
|
Motion in a plane. Cases of uniform velocity and uniform acceleration-projectile motion. Uniform circular motion. |
|
|
Laws of Motion |
Intuitive concept of force. Inertia, Newton's first law of motion; momentum and Newton's second law of motion; impulse; Newton'sthird law of motion |
|
Law of conservation of linear momentum and its applications. |
|
|
Equilibrium of concurrent forces. Static and kinetic friction, laws of friction, rolling friction, lubrication. |
|
|
Dynamics of uniform circular motion: Centripetal force, examples of circular motion (vehicle on a level circular road, vehicle on a banked road) |
|
|
Work, Energy, and Power |
Work done by a constant force and a variable force; kinetic energy, work-energy theorem, power |
|
The notion of potential energy, the potential energy of a spring, conservative forces: non-conservative forces: motion in a vertical circle, elastic and inelastic collisions in one and two dimensions. |
|
|
Motion of System of Particles and Rigid Body |
System of Particles and Rotational Motion Centre of mass of a two-particle system, momentum conservation, and center of mass motion. |
|
Centre of mass of a rigid body; center of mass of a uniform rod. |
|
|
Moment of a force, torque, angular momentum, laws of conservation of angular momentum, and its applications. |
|
|
Equilibrium of rigid bodies, rigid body rotation and equations of rotational motion, comparison of linear and rotational motions. |
|
|
Moment of inertia, radius of gyration. Values of moments of inertia, for simple geometrical objects (no derivation) |
|
|
Gravitation |
Kepler'slaws of planetary motion. The universal law of gravitation. |
|
Acceleration due to gravity and its variation with altitude and depth. |
|
|
Gravitational potential energy and gravitational potential. Escape velocity. Orbital velocity of a satellite. |
|
|
Properties of Bulk Matter |
Mechanical Properties of Solids Elastic behavior, Stress-strain relationship, Hooke's law, Young's modulus, bulk modulus, shear modulus of rigidity, (qualitative idea only), Poisson'sratio; elastic energy |
|
Mechanical Properties of Fluids Pressure due to a fluid column; Pascal'slaw and its applications. (hydraulic lift and hydraulic brakes), Effect of gravity on fluid pressure |
|
|
Viscosity, Stokes' law, terminal velocity, streamline and turbulent flow, critical velocity. Bernoulli'stheorem and its simple applications. |
|
|
Surface energy and surface tension, angle of contact, excess of pressure across a curved surface, application of surface tension ideas to drops, bubbles, and capillary rise. |
|
|
Thermal Properties of Matter Heat, temperature, thermal expansion; thermal expansion of solids, liquids, and gases, Anomalous expansion of water; specific heat capacity; Cp, Cv - calorimetry; change of state -latent heat capacity. |
|
|
Heat transfer-conduction, convection and radiation, thermal conductivity, Qualitative ideas of Blackbody radiation, Wein's displacement Law, Stefan'slaw. |
|
|
Thermodynamics |
Thermal equilibrium and definition of temperature (zeroth law of thermodynamics).Heat, work, and internal energy. Firstlaw of thermodynamics |
|
The second law of thermodynamics: gaseous state of matter, change of condition of gaseous state - isothermal, adiabatic, reversible, irreversible, and cyclic processes |
|
|
Behavior of Perfect Gases and Kinetic Theory of Gases |
Kinetic Theory Equation of state of a perfect gas, work done in compressing a gas. |
|
Kinetic theory of gases - assumptions, concept of pressure. Kinetic interpretation of temperature; rms speed of gas molecules; degrees of freedom, law of equipartition of energy (statement only) and application to specific heat capacities of gases; concept of mean free path, Avogadro's number. |
|
|
Oscillations and Waves |
Oscillations Periodic motion - time period, frequency, displacement as a function of time. Periodic functions and their application |
|
Simple harmonic motion (S.H.M) and its equation; phase; oscillations of a loaded spring-restoring force and force constant; energy in S.H.M. Kinetic and potential energies; simple pendulum derivation of expression for its time period. |
|
|
Waves Wave motion. Transverse and longitudinal waves, speed of traveling wave. Displacement relation for a progressive wave. Principle of superposition of waves, reflection of waves, standing waves in strings and organ pipes, fundamental mode and harmonics, Beats. |
|
|
Physics XII |
|
|
Electrostatics |
Electric Charges and Fields Electric Charges; Conservation of charge, Coulomb’s law force between two point charges, forces between multiple charges; superposition principle, and continuous charge distribution. |
|
Electric field, electric field due to a point charge, electric field lines, electric dipole, electric field due to a dipole, torque on a dipole in a uniform electric field. |
|
|
Electric flux, statement of Gauss’s theorem and its applications to find field due to infinitely long straight wire, uniformly charged infinite plane sheet, and uniformly charged thin spherical shell (field inside and outside). |
|
|
Electrostatic Potential and Capacitance Electric potential, potential difference, electric potential due to a point charge, a dipole, and a system of charges; equipotential surfaces, the electrical potential energy of a system of two-point charges, and of electric dipole in an electrostatic field. |
|
|
Conductors and insulators, free charges, and bound charges inside a conductor. Dielectrics and electric polarization, capacitors, and capacitance, a combination of capacitors in series and in parallel, the capacitance of a parallel plate capacitor with and without dielectric medium between the plates, energy stored in a capacitor. (no derivation, formulae only). |
|
|
Current Electricity |
Electric current, the flow of electric charges in a metallic conductor, drift velocity, mobility and their relation with electric current; Ohm’s law, V-I characteristics (linear and non-linear), electrical energy and power, electrical resistivity, and conductivity, the temperature dependence of resistance |
|
The internal resistance of a cell, potential difference and emf of a cell, combination of cells in series and in parallel. |
|
|
Kirchhoff’slaws, Wheatstone bridge. |
|
|
Magnetic Effects of Current and Magnetism |
Moving Charges and Magnetism Concept of the magnetic field, Oersted’s experiment. |
|
Biot - Savart law and its application to the current carrying circular loop. |
|
|
Ampere’s law and its applications to infinitely long straight wire. Straight solenoid (only qualitative treatment), Force on a moving charge in uniform magnetic and electric fields. |
|
|
Force on a current-carrying conductor in a uniform magnetic field. The force between two parallel current-carrying conductors- definition of ampere. Torque experienced by a current loop in a uniform magnetic field; Current loop as a magnetic dipole and its magnetic dipole moment. Moving coil galvanometer-its current sensitivity and conversion to ammeter and voltmeter |
|
|
Magnetism and Matter Bar magnet, bar magnet as an equivalent solenoid, (qualitative treatment only)Magnetic field intensity due to a magnetic dipole (bar magnet) along its axis and perpendicular to its axis. (qualitative treatment only), Torque on a magnetic dipole (bar magnet) in a uniform magnetic field; (qualitative treatment only), magnetic field Lines; Magnetic properties of materials-Para-, dia- and ferromagnetic substances, with examples Magnetization of materials, the effect of temperature on magnetic properties. |
|
|
Electromagnetic Induction and Alternating Currents |
Electromagnetic Induction Faraday’s laws induced EMF and current; Lenz’s Law, Self and mutual induction. |
|
Alternating Current Alternating currents, peak and rms value of alternating current/voltage; reactance and impedance; LCR series circuit, (phasors only), resonance; power in AC circuits, wattles current |
|
|
AC generator and Transformer. |
|
|
Electromagnetic waves |
The basic idea of displacement current. Electromagnetic waves and their characteristics Transverse nature of electromagnetic waves. (Qualitative ideas only). |
|
Electromagnetic spectrum (radio waves, microwaves, infrared, visible, ultraviolet, X-rays, gamma rays) including elementary facts about their uses. |
|
|
Optics |
Ray Optics and Optical Instruments. Ray Optics: Reflection of light, spherical mirrors, mirror formula. Refraction of light, total internal reflection and optical fibers, refraction at spherical surfaces, lenses, thin lens formula, lens maker’s formula. Magnification, power of a lens, combination of thin lenses in contact. Refraction of light through a prism. |
|
Optical instruments: Microscopes and astronomical telescopes (reflecting and refracting) and their magnifying powers. |
|
|
Wave Optics Wave optics: Wave front and Huygens principle, reflection and refraction of plane wave at a plane surface using wave fronts. Proof of laws of reflection and refraction using Huygens principle. Interference, Young's double slit experiment and expression for fringe width, (No derivation final expression only) coherent sources and sustained interference of light. Diffraction due to a single slit, width of central maximum, (qualitative treatment only). |
|
|
Dual Nature of Matter and Radiation |
Dual nature of radiation. Photoelectric effect, Hertz and Lenard's observations; Einstein’s photoelectric equation-particle nature of light. Experimental study of photoelectric effect. |
|
Matter waves-wave nature of particles, de Broglie relation |
|
|
Atoms & Nuclei |
Atoms Alpha-particle scattering experiment; Rutherford’s model of atom; Bohr model, of the hydrogen atom, Expression for the radius of nth possible orbit, velocity and energy of electron in this orbit, hydrogen line spectra (qualitative treatment only). |
|
Nuclei Composition and size of nucleus, nuclear force Mass-energy relation, mass defect; binding energy per nucleon and its variation with mass number; nuclear fission, nuclear fusion |
|
|
Electronic Devices |
Semiconductor Electronics Materials, Devices, and Simple Circuits Energy bands in solids conductors, insulators, and semiconductors; (Qualitative ideas only) Intrinsic and extrinsic semiconductors- p and n-type, p-n junction Semiconductor diode– I-V characteristics in forward and reverse bias, application of junction diode - diode as a rectifier |
Chemistry
In this subject, you will understand the reactions, chemical bonding, organic and inorganic chemistry, and other related topics.
|
Chemistry XI |
|
|
Units |
Topics |
|
Some Basic Concepts of Chemistry |
General Introduction: Importance and scope of Chemistry. Nature of matter, laws of chemical combination, Dalton's atomic theory: the concept of elements, atoms, and molecules. Atomic and molecular masses, mole concept and molar mass, percentage composition, empirical and molecular formula, chemical reactions, stoichiometry, and calculations based on stoichiometry |
|
Structure of Atom |
Discovery of Electron, Proton and Neutron, atomic number, isotopes and isobars. Thomson's model and its limitations. Rutherford's model and its limitations, Bohr's model and its limitations, concept of shells and subshells, dual nature of matter and light, de Broglie's relationship, Heisenberg uncertainty principle, concept of orbitals, quantum numbers, shapes of s, p and d orbitals, rules for filling electrons in orbitals - Aufbau principle, Pauli's exclusion principle and Hund's rule, electronic configuration of atoms, stability of half-filled and completely filled orbitals. |
|
Classification of Elements and Periodicity in Properties |
Significance of classification, a brief history of the development of the periodic table, modern periodic law and the present form of the periodic table, periodic trends in properties of elements -atomic radii, ionic radii, inert gas radii, Ionization enthalpy, electron gain enthalpy, electronegativity, valency. Nomenclature of elements with atomic number greater than 100. |
|
Chemical Bonding and Molecular Structure |
Valence electrons, ionic bond, covalent bond, bond parameters, Lewis’s structure, polar character of covalent bond, covalent character of ionic bond, valence bond theory, resonance, geometry of covalent molecules, VSEPR theory, concept of hybridization, involving s, p and d orbitals and shapes of some simple molecules, molecular orbital theory of homonuclear diatomic molecules (qualitative idea only), Hydrogen bond. |
|
Chemical Thermodynamics |
Concepts of Systems and types of systems, surroundings, work, heat, energy, extensive and intensive properties, and state functions. The first law of thermodynamics -internal energy and enthalpy, heat capacity, and specific heat, measurement of ΔU and ΔH, Hess's law of constant heat summation, enthalpy of bond dissociation, combustion, formation, atomization, sublimation, phase transition, ionization, solution, and dilution. Second law of Thermodynamics (brief introduction) Introduction of entropy as a state function, Gibb's energy change for spontaneous and non-spontaneous processes, criteria for equilibrium. Third law of thermodynamics (brief introduction). |
|
Equilibrium |
Equilibrium in physical and chemical processes, dynamic nature of equilibrium, the law of mass action, equilibrium constant, factors affecting equilibrium - Le Chatelier's principle, ionic equilibrium- ionization of acids and bases, strong and weak electrolytes, degree of ionization, ionization of polybasic acids, acid strength, the concept of pH, hydrolysis of salts (elementary idea), buffer solution, Henderson Equation, solubility product, common ion effect (with illustrative examples). |
|
Redox Reactions |
Concept of oxidation and reduction, redox reactions, oxidation number, balancing redox reactions, in terms of loss and gain of electrons and change in oxidation number, applications of redox reactions. |
|
Organic Chemistry - Some Basic Principles and Techniques |
General introduction, methods of purification, qualitative and quantitative analysis, classification, and IUPAC nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electrometric effect, resonance, and hyperconjugation. Homolytic and heterolytic fission of a covalent bond: free radicals, carbocations, carbanions, electrophiles and nucleophiles, types of organic reactions. |
|
Hydrocarbons |
Classification of Hydrocarbons Aliphatic Hydrocarbons: Alkanes- Nomenclature, isomerism, conformation (ethane only), physical properties, chemical reactions including free radical mechanisms of halogenation, combustion, and pyrolysis. Alkenes- Nomenclature, the structure of double bond (ethene), geometrical isomerism, physical properties, methods of preparation, chemical reactions: addition of hydrogen, halogen, water, hydrogen halides (Markovnikov's addition and peroxide effect), ozonolysis, oxidation, mechanism of electrophilic addition. Alkynes- Nomenclature, the structure of triple bond (ethyne), physical properties, methods of preparation, chemical reactions: acidic character of alkynes, addition reaction of - hydrogen, halogens, hydrogen halides, and water. |
|
Aromatic Hydrocarbons: Introduction, IUPAC nomenclature, benzene: resonance, aromaticity, chemical properties: mechanism of electrophilic substitution. Nitration, sulphonation, halogenation, Friedel Craft's alkylation and acylation, directive influence of the functional group in monosubstituted benzene. Carcinogenicity and toxicity. |
|
|
Chemistry XII |
|
|
Solutions |
Types of solutions, expression of concentration of solutions of solids in liquids, solubility of gases in liquids, solid solutions, Raoult's law, colligative properties - relative lowering of vapor pressure, elevation of boiling point, depression of freezing point, osmotic pressure, determination of molecular masses using colligative properties, abnormal molecular mass, Van't Hoff factor. |
|
Electrochemistry |
Redox reactions, EMF of a cell, standard electrode potential, Nernst equation and its application to chemical cells, Relation between Gibbs energy change and EMF of a cell, conductance in electrolytic solutions, specific and molar conductivity, variations of conductivity with concentration, Kohlrausch's Law, electrolysis and law of electrolysis (elementary idea), dry cell-electrolytic cells and Galvanic cells, lead accumulator, fuel cells, corrosion. |
|
Chemical Kinetics |
Rate of a reaction (Average and instantaneous), factors affecting rate of reaction: concentration, temperature, catalyst; order and molecularity of a reaction, rate law and specific rate constant, integrated rate equations and half-life (only for zero and first order reactions), concept of collision theory (elementary idea, no mathematical treatment), activation energy, Arrhenius equation |
|
d and f Block Elements |
General introduction, electronic configuration, occurrence and characteristics of transition metals, general trends in properties of the first-row transition metals – metallic character, ionization enthalpy, oxidation states, ionic radii, color, catalytic property, magnetic properties, interstitial compounds, alloy formation, preparation and properties of K2Cr2O7 and KMnO4. |
|
Lanthanoids - Electronic configuration, oxidation states, chemical reactivity, and lanthanoid contraction and its consequences |
|
|
Actinoids - Electronic configuration, oxidation states, and comparison with lanthanoids. |
|
|
Coordination Compounds |
Coordination compounds - Introduction, ligands, coordination number, color, magnetic properties and shapes, IUPAC nomenclature of mononuclear coordination compounds. Bonding, Werner's theory, VBT, and CFT; structure and stereoisomerism, importance of coordination compounds (in qualitative analysis, extraction of metals and biological system). |
|
Haloalkanes and Haloarenes |
Haloalkanes: Nomenclature, nature of C–X bond, physical and chemical properties, optical rotation mechanism of substitution reactions |
|
Haloarenes: Nature of C–X bond, substitution reactions (Directive influence of halogen in monosubstituted compounds only). Uses and environmental effects of - dichloromethane, trichloromethane, tetrachloromethane, iodoform, freons, DDT. |
|
|
Alcohols, Phenols and Ethers |
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only), identification of primary, secondary, and tertiary alcohols, mechanism of dehydration, and uses with special reference to methanol and ethanol. |
|
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophilic substitution reactions, uses of phenols. |
|
|
Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses. |
|
|
Aldehydes, Ketones and Carboxylic Acids |
Aldehydes and Ketones: Nomenclature, nature of carbonyl group, methods of preparation, physical and chemical properties, mechanism of nucleophilic addition, reactivity of alpha hydrogen in aldehydes, uses. |
|
Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical and chemical properties; uses. |
|
|
Amines |
Amines: Nomenclature, classification, structure, methods of preparation, physical and chemical properties, uses, and identification of primary, secondary, and tertiary amines. |
|
Diazonium salts: Preparation, chemical reactions and importance in synthetic organic chemistry. |
|
|
Biomolecules |
Carbohydrates - Classification (aldoses and ketoses), monosaccharides (glucose and fructose), D-L configuration oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen); Importance of carbohydrates. |
|
Proteins -Elementary idea of - amino acids, peptide bonds, polypeptides, proteins, structure of proteins - primary, secondary, tertiary structure and quaternary structures (qualitative idea only), denaturation of proteins; enzymes. Hormones - Elementary idea excluding structure. |
|
|
Vitamins - Classification and functions. |
|
|
Nucleic Acids: DNA and RNA. |
|
Mathematics
Maths is a vast subject that covers topics like circles, triangles, trigonometry, and other important topics. Make sure you cover them all to achieve good grades.
|
Mathematics XI |
|
|
Units |
Topics |
|
Sets and Functions |
Sets Sets and their representations, Empty sets, Finite and Infinite sets, Equal sets, Subsets, and Subsets of a set of real numbers especially intervals (with notations). Universal set. Venn diagrams. Union and Intersection of sets. Difference of sets. Complement of a set. Properties of Complement. |
|
Relations & Functions |
|
|
Trigonometric Functions |
|
|
Algebra |
Complex Numbers and Quadratic Equations Need for complex numbers, especially √−𝟏, to be motivated by inability to solve some of the quadratic equations. Algebraic properties of complex numbers. Argand plane |
|
Linear Inequalities Linear inequalities. Algebraic solutions of linear inequalities in one variable and their representation on the number line. |
|
|
Permutations and Combinations Fundamental principle of counting. Factorial n. (n!) Permutations and combinations, derivation of Formulae for nPr and nCr and their connections, simple applications |
|
|
Binomial Theorem |
|
|
Sequence and Series |
|
|
Coordinate Geometry |
Straight Lines Brief recall of two-dimensional geometry from earlier classes. The slope of a line and the angle between two lines. Various forms of equations of a line: parallel to the axis, point-slope form, slope-intercept form, two-point form, intercept form, and Distance of a point from a line. |
|
Conic Sections Sections of a cone: circles, ellipse, parabola, hyperbola, a point, a straight line, and a pair of intersecting lines as a degenerated case of a conic section. Standard equations and simple properties of parabola, ellipse, and hyperbola. Standard equation of a circle. |
|
|
Introduction to Three-dimensional Geometry Coordinate axes and coordinate planes in three dimensions. Coordinates of a point. Distance between two points. |
|
|
Calculus |
Limits and Derivatives Derivative introduced as rate of change both as that of distance function and geometrically. Intuitive idea of limit. Limits of polynomials and rational functions trigonometric, exponential, and logarithmic functions. The definition of derivative relates to the slope of the tangent of the curve, derivative of sum, difference, product, and quotient of functions. Derivatives of polynomial and trigonometric functions. |
|
Statistics and Probability |
Statistics Measures of Dispersion: Range, Mean deviation, variance, and standard deviation of ungrouped/grouped data. |
|
Probability Events; occurrence of events, ‘not’, ‘and’ and ‘or’ events, exhaustive events, mutually exclusive events, Axiomatic (set theoretic) probability, connections with other theories of earlier classes. Probability of an event, probability of ‘not’, ‘and’ and ‘or’ events. |
|
|
Mathematics XII |
|
|
Relations and Functions |
Relations and Functions Types of relations: reflexive, symmetric, transitive, and equivalence relations. One-to-one and onto functions. |
|
Inverse Trigonometric Functions Definition, range, domain, principal value branch. Graphs of inverse trigonometric functions. |
|
|
Algebra |
Matrices Concept, notation, order, equality, types of matrices, zero and identity matrix, transpose of a matrix, symmetric and skew-symmetric matrices. Operations on matrices: Addition and multiplication and multiplication with a scalar. Simple properties of addition, multiplication, and scalar multiplication. Noncommutativity of multiplication of matrices and existence of non-zero matrices whose product is the zero matrix (restricted to square matrices of order 2). Invertible matrices and proof of the uniqueness of inverse, if it exists; (Here all matrices will have real entries). |
|
Determinants |
|
|
Calculus |
Continuity and Differentiability Continuity and differentiability, chain rule, a derivative of inverse trigonometric functions,𝑙𝑖𝑘𝑒 sin−1 𝑥, cos−1 𝑥 and tan−1 𝑥, derivative of implicit functions. Concept of exponential and logarithmic functions. Derivatives of logarithmic and exponential functions. Logarithmic differentiation is a derivative of functions expressed in parametric forms. Second-order derivatives. |
|
Applications of Derivatives Applications of derivatives: rate of change of quantities, increasing/decreasing functions, maxima, and minima (first derivative test motivated geometrically and second derivative test given as a provable tool). Simple problems (that illustrate basic principles and understanding of the subject as well as real-life situations). |
|
|
Integrals |
|
|
Applications of the Integrals Applications in finding the area under simple curves, especially lines, circles/ parabolas/ellipses (in standard form only) |
|
|
Differential Equations |
|
|
Vectors and Three-Dimensional Geometry |
Vectors Vectors and scalars, magnitude and direction of a vector. Direction cosines and direction ratios of a vector. Types of vectors (equal, unit, zero, parallel, and collinear vectors), position vector of a point, negative of a vector, components of a vector, addition of vectors, multiplication of a vector by a scalar, position vector of a point dividing a line segment in a given ratio. Definition, Geometrical Interpretation, properties and application of scalar (dot) product of vectors, vector (cross) product of vectors. |
|
Three - dimensional Geometry Direction cosines and direction ratios of a line joining two points. Cartesian equation and vector equation of a line, skew lines, shortest distance between two lines. The angle between two lines |
|
|
Linear Programming |
Introduction, related terminology such as constraints, objective function, optimization, graphical method of solution for problems in two variables, feasible and infeasible regions (bounded or unbounded), feasible and infeasible solutions, optimal feasible solutions (up to three non-trivial constraints). |
|
Probability |
Conditional probability, multiplication theorem on probability, independent events, total probability, Bayes’ theorem, Random variable, and its probability distribution, mean of the random variable. |
Eligibility Criteria For The Engineering Field Of The COMEDK Exam
COMEDK exam follows an eligibility criteria that you should be aware of. We have listed the points of eligibility below for your reference.
- To apply for COMEDK UGET, you should be a citizen of India.
- You should have passed the 10+2 class from the recognized board of the state and the last two years should be from the science stream which includes Physics, Chemistry, and Mathematics with English as a compulsory subject.
- If you belong to the general category, then you should have passed Physics, Chemistry, and Mathematics with almost 45% marks individually.
- In case, you belong to the Schedule Caste, Schedule Tribe, and OBC categories, then you should have passed the science stream with 40% in each subject.
- You must have Physics and Mathematics as the compulsory subjects and Biology, Chemistry, Biotechnology, Computer Science, and Electronics have to be one of the optional subjects.
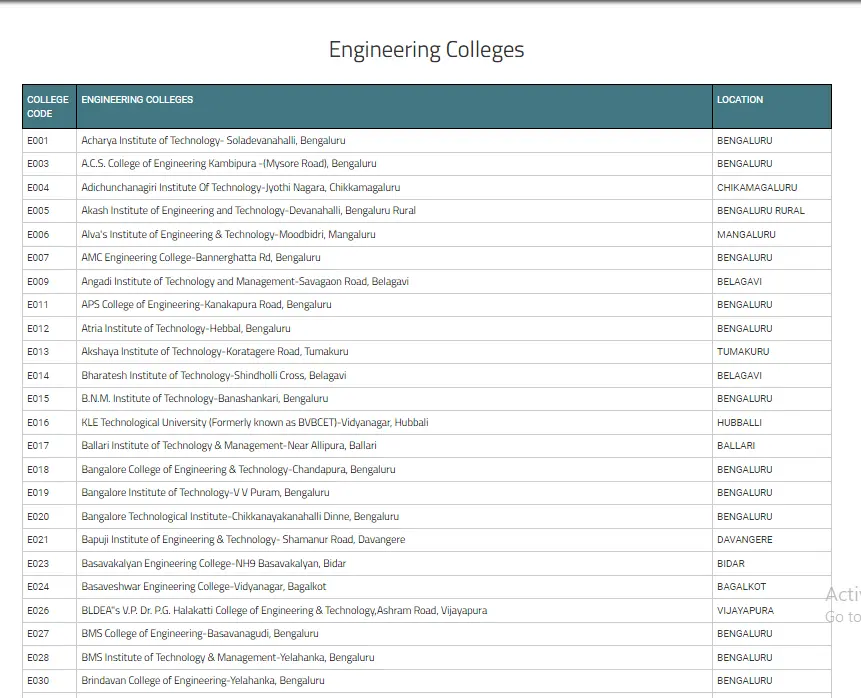
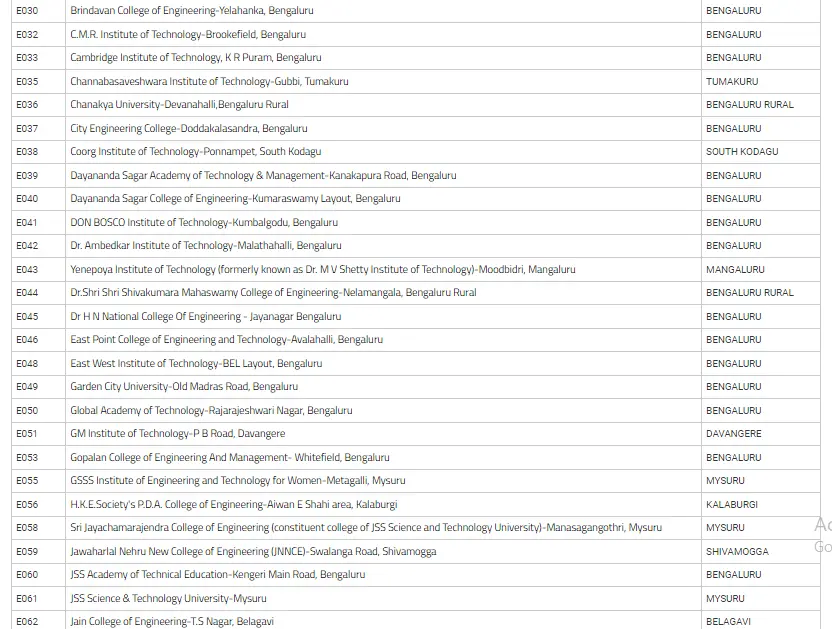
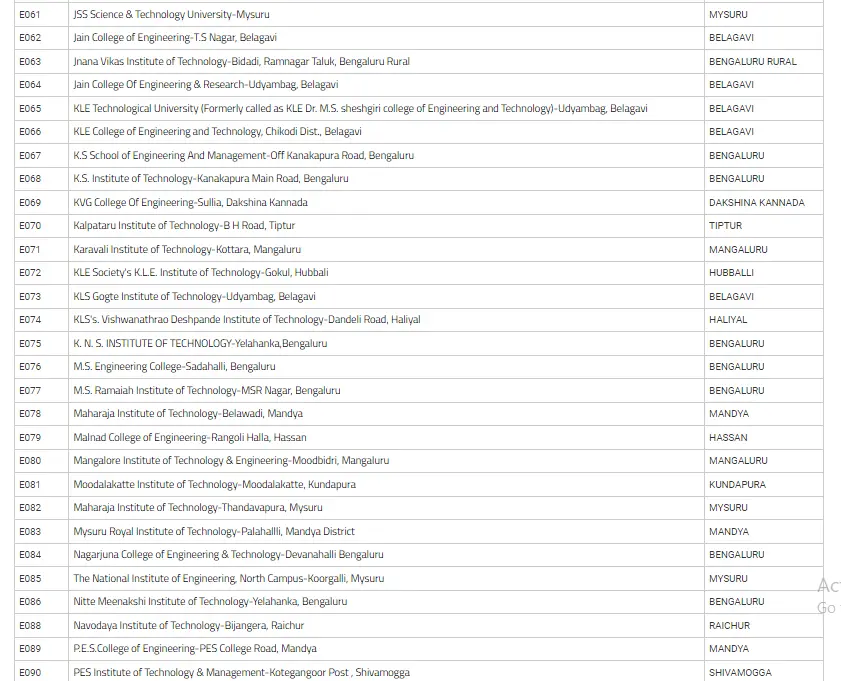
List Of Colleges For Engineering Field Through COMEDK UGET
In the below image, we have mentioned the list of colleges of Engineering with their location in Karnataka state.

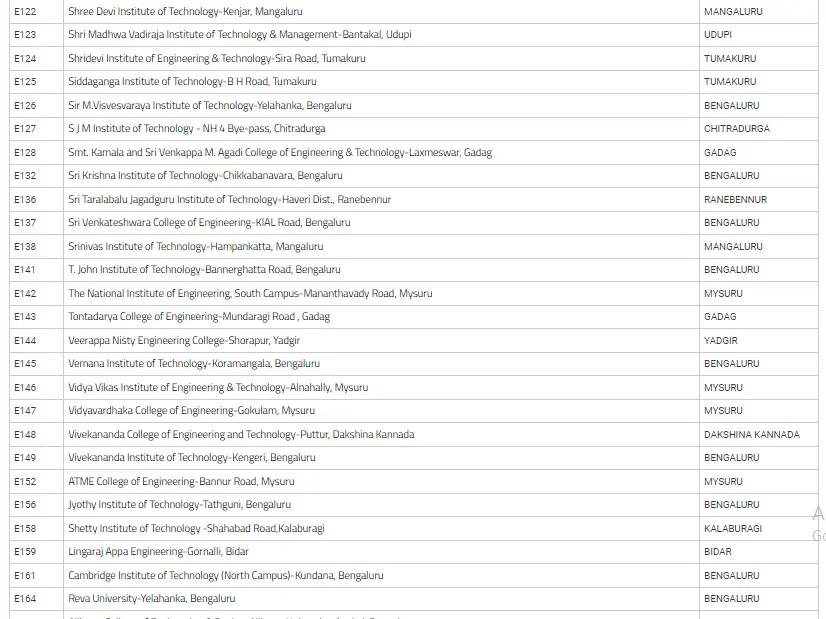
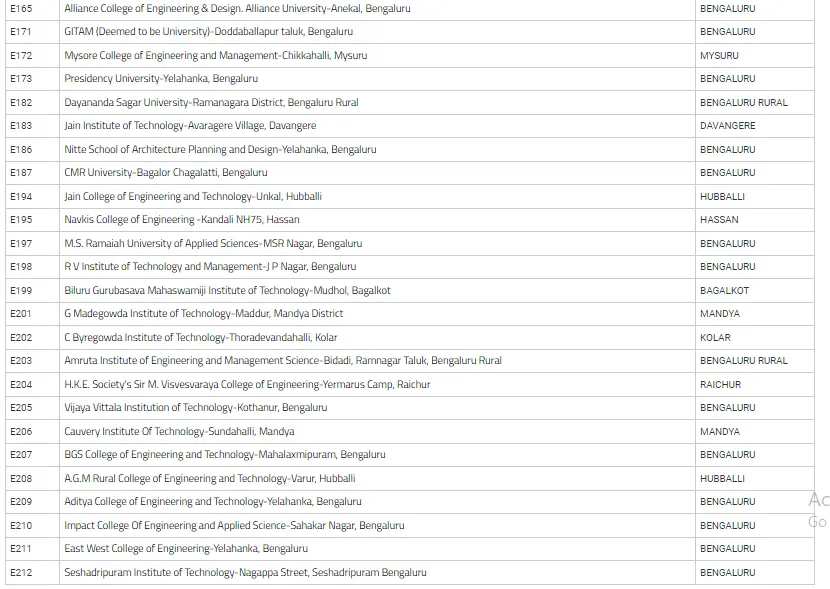
How To Prepare For The Exam By Using The COMEDK Mock Test?
You can use our COMEDK Mock Test series to get a comprehensive idea of all the important factors and prepare effectively for the examination.
- Understand the Exam Format: Take your time to understand the syllabus and exam format of the exam. With the COMEDK Mock Test free, you can get familiar with the types of Questions that reflect the actual test. You must check out the different PYPs to note down the frequently asked topics and analyze the difficulty level of the Questions.
- Organized Study Material: Before beginning with the COMEDK Mock Test 2026, sit with all the necessary items required to attempt the test. Collect your study notes, reference books, and other textbooks to aid your preparation. Make sure to divide the specific time slots for all subjects and cover every topic of the subject.
- Group Studies: The best advantage of attempting the COMEDK Online Mock Test with your study group is that it will help you solve the Questions from different perspectives. If one does not understand the Question, then another classmate could help them to clear their doubts.
- Analyzing the performance: Keep a regular check on your performance in the COMEDK Mock Test series by writing down the marks you obtain in every test so you can track your progress. With the improvement in scores, you would know that you are getting better at solving the Mock Test and can also identify the areas that require more improvement.
How To Choose The Right Engineering Specialization After The COMEDK Exam?
Selecting the correct engineering specialization after clearing the exam will shape your future. Here, we have given the guide by which you can choose your specialization with more accuracy.
- Know your passion: Identify your personal interest and what you are more passionate about in the field of engineering. If you are interested in gadgets and electronics, then you can choose electrical and computer engineering. You can attempt any of the specialized COMEDK Mock Tests to clear your doubts about the field. You can check if your strengths fit best with your chosen specialization.
- Understand different fields of Engineering: Research all the engineering fields such as mechanical, civil, chemical, electrical, and biomedical engineering. Read all the related information on the specific field to know more about each of them. Check which of the fields could give you a great career path in the future and have a higher market value.
- Choosing the Career Prospect: If you have chosen one field, check if it has better demand in the industry or not because some of the job positions have more stability than others. Start your research with the growth of the industry and the employment rates increased per year. With this, you can make smart decisions with a high salary package and extra perks in the selected field.
- Advice from Professionals: If you have selected the field of your interest and you still are doubtful about your decision, try to seek advice from a senior who is already pursuing that specific field or has just passed to know the latest update of the course. You can also ask the professionals who have mastered and are settled at this time so you can get a clearer view of your future responsibilities.
Important Topics And Their Weightage In The COMEDK Exam
In the below table, we have mentioned some important topics that you should cover as they contain a high weightage in the exam.
|
Physics |
|
|
Topic |
Weightage |
|
Optics |
10-12% |
|
Mechanics |
15-18% |
|
Magnetic Effects of Current and Magnetism |
6-8% |
|
Electromagnetic Induction and Alternating Currents |
5-7% |
|
Electronic Devices: |
5-7% |
|
Chemistry |
|
|
Solutions |
5-7% |
|
p-Block Elements |
5-7% |
|
Chemical Kinetics |
4-6% |
|
Solid State |
5-7% |
|
Aldehydes, Ketones, and Carboxylic Acid |
4-6% |
|
Mathematics |
|
|
Limit, Continuity, and Differentiability |
8-10% |
|
Coordinate Geometry |
10-12% |
|
Statistics and Probability |
8-10% |
|
Integral Calculus |
8-10% |
|
Trigonometry |
7-9% |







 Profile
Profile Signout
Signout










 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










