CBSE 12th exams are going on and your Chemistry paper is on 27th February 2024. You have only a few hours left for the Chemistry exam.
Therefore, we have introduced students to the best last minute revision tool – the important Topic-Wise Most Important Questions!
This article is for you for CBSE Class 12 Chemistry (Multiple Choice, Assertion-Reason, Case-Based Question, Conceptual Question, Short/Long Question) 4 or 5 marks questions are long answer type questions where students have to answer in the final. A detailed answer with additional information is required to score correctly.
The important questions given to you here are essential from the point of view of CBSE Class 12 Board Exam 2024 and hence all the prospective candidates of the exam should practice these questions. We have mentioned all the authentic sources to get the most important questions from each chapter.
In this, every important question has been sorted and collected, which is very important for your paper, so that the student can score good marks in less time. Answers to all the questions are given together.
CBSE Class 12th Chemistry Important Questions 2023-24
CBSE Class 12 Study Materials
| CBSE Class 12 Syllabus 2023-24 | CBSE Class 12 Previous Year Papers |
| NCERT Books For Class 12 Books | NCERT Class 12 Solutions |
| CBSE Class 12 Full Study Material | CBSE Class 12 Sample Paper 2023-24 |
Multiple Choice Question
1. Which of the following is an example of a solid solution?
(a) Sea water
(b) Sugar solution
(c) Smoke
(d) 22 carat gold
Ans. (d) 22 carat gold
2. Value of Henry's constant KH
(a) increases with decrease in temperature.
(b) decreases with increase in temperature.
(c) increases with increase in temperature.
(d) remains constant.
Ans. (c) increases with increase in temperature.
3. Which of the following solutions of KCI will have the highest value of specific conductance?
(a) 0.5 M
(b) 0.01 M
(c) 0.1 M
(d) 1.0 M
Ans. (c) 0.1 M
4. In fuel cell
(a) chemical energy is converted to electrical energy.
(b) energy of combustion of fuel is converted to chemical energy.
(c) energy of combustion of fuel is converted to electrical energy.
(d) electrical energy is converted to chemical energy.
Ans. (c) energy of combustion of fuel is converted to electrical energy.
5. Which of the following statements are applicable to a balanced chemical equation of an elementary reaction?
(a) Order is same as molecularity.
(b) Order is less than the molecularity.
(c) Order is greater than the molecularity.
(d) Molecularity can be zero.
Ans. (a) Order is same as molecularity.
6. The role of a catalyst is to change.....................
(a) gibbs energy of reaction
(b) enthalpy of reaction
(c) activation energy of reaction
(d) equilibrium constant
Ans. (c) activation energy of reaction
7. Which of the following is the reason for Zinc not exhibiting variable oxidation state?
(a) Inert pair effect
(b) Completely filled 3d subshell
(c) Completely filled 4s subshell
(d) Common ion effect
Ans. (b) Completely filled 3d subshell
8. Which of the following characteristics of transition metals is associated with their catalytic activity?
(a) Paramagnetic nature
(b) Colour of hydrated ions
(c) High enthalpy of atomisation
(d) Variable oxidation states
Ans. (d) Variable oxidation states
9. Which of the following species is not expected to be a ligand?
(a) NO
(b) NH+4
(c) NH2CH2CH2NH2
(d) CO
Ans. (b) NH+4
10. EDTA is a
(a) monodentate ligand
(b) bidentate ligand
(c) ambidentate ligand
(d) hexadentate ligand
Ans. (d) hexadentate ligand
11. Ethylene chloride and ethylidene chloride are isomers. Identify the correct statements.
(i) Both the compounds form same product on treatment with alcoholic KOH.
(ii) Both the compounds form same product on treatment with aq. NaOH.
(iii) Both the compounds form same product on reduction.
(iv) Both the compounds are optically active.
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (i) and (ii)
Ans. (a) (i) and (iii)
12. Haloalkanes contain halogen atom(s) attached to the sp³ hybridised carbon atom of an alkyl group. Identify haloalkane from the following compounds.
(a) 2-Bromopentane
(b) Vinyl chloride (chloroethene)
(c) 2-Chloroacetophenone
(d) Chlorobenzene
Ans. (a) 2-Bromopentane
13. The C-O-H bond angle in alcohol is
(a) slightly greater than 109°28'.
(b) slightly less than 109°28'.
(c) slightly greater than 120°.
(d) slightly less than 120°.
Ans. (b) slightly less than 109°28'.
14. Williamson's synthesis of preparing dimethyl ether is an:
(a) SNl reaction
(b) Elimination reaction
(c) SN2 reaction
(d) Nucleophilic addition reaction
Ans. (b) Elimination reaction
15. The formation of cyanohydrin from propanone is which type of reaction?
(a) Electrophilic substitution
(b) Nucleophilic substitution
(c) Electrophilic addition
(d) Nucleophilic addition
Ans. (d) Nucleophilic addition
16. Iodoform test is not given by
(a) Ethanol
(b) Ethanal
(c) Pentan-2-one
(d) Pentan-3-one
Ans. (d) Pentan-3-one
17. Out of the following, the strongest base in aqueous solution is
(a) Methylamine
(b) Dimethylamine
(c) Trimethylamine
(d) Aniline
Ans. (b) Dimethylamine
18. The action of nitrous acid on ethylamine gives mainly :
(a) ethyl nitrite
(b) ethyl alcohol
(c) nitroethane
(d) ethane
Ans. (a) ethyl nitrite
19. Which of the following reactions of glucose can be explained only by its cyclic structure?
(a) Glucose forms pentaacetate.
(b) Glucose reacts with hydroxylamine to form an oxime.
(c) Pentaacetate of glucose does not react with hydroxylamine.
(d) Glucose is oxidised by nitric acid to gluconic acid.
Ans. (c) Pentaacetate of glucose does not react with hydroxylamine.
20. Nucleosides are composed of
(a) a pentose sugar and phosphoric acid.
(b) a nitrogenous base and phosphoric acid.
(c) a nitrogenous base and a pentose sugar.
(d) a nitrogenous base, a pentose sugar and phosphoric acid.
Ans.(c) a nitrogenous base and a pentose sugar.
Assertion-Reason Question
In the following questions, two statements are given-one labeled Assertion (A) and the other labeled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
(a) Both Assertion (A) and Reason (R) are correct statements, and Reason (R) is the correct explanation of the Assertion (A).
(b) Both Assertion (A) and Reason (R) are correct statements, but Reason (R) is not the correct explanation of the Assertion (A).
(c) Assertion (A) is correct, but Reason (R) is incorrect statement.
(d) Assertion (A) is incorrect, but Reason (R) is correct statement.
1. Assertion (A): Cryoscopic constant depends on nature of solvent.
Reason (R): Cryoscopic constant is a universal constant.
Ans. (c) Assertion (A) is correct, but Reason (R) is incorrect statement.
2. Assertion (A): Mercury cell does not give steady potential.
Reason (R): In the cell reaction, ions are not involved in solution.
Ans. (d) Assertion (A) is incorrect, but Reason (R) is correct statement.
3. Assertion (A): For each ten degree rise of temperature the specific rate constant is nearly doubled.
Reason (R): Energy-wise distribution of molecules in a gas is an experimental function of temperature.
Ans. (b) Both Assertion (A) and Reason (R) are correct statements, but Reason (R) is not the correct explanation of the Assertion (A).
4. Assertion (A): Zn, Cd and Hg cannot be regarded as transition elements.
Reason (R): These elements do not belong to the d-block of the periodic table.
Ans. (c) Assertion (A) is correct, but Reason (R) is incorrect statement.
5. Assertion (A): Low spin tetrahedral complexes are rarely observed.
Reason (R): Crystal field splitting energy is less than pairing energy for tetrahedral complexes.
Ans. (a) Both Assertion (A) and Reason (R) are correct statements, and Reason (R) is the correct explanation of the Assertion (A).
6. Assertion (A): Aryl halides undergo nucleophilic substitution reactions with ease.
Reason (R): The carbon halogen bond in aryl halides has partial double bond character.
Ans. (d) Assertion (A) is incorrect, but Reason (R) is correct statement.
7. Assertion (A): Phenol is more reactive than benzene towards electrophilic substitution reaction.
Reason (R): In the case of phenol, the intermediate carbocation is more resonance stabilized.
Ans. (a) Both Assertion (A) and Reason (R) are correct statements, and Reason (R) is the correct explanation of the Assertion (A).
8. Assertion (A): Carboxylic acids are more acidic than phenols.
Reason (R): Phenols are ortho and para directing.
Ans. (b) Both Assertion (A) and Reason (R) are correct statements, but Reason (R) is not the correct explanation of the Assertion (A).
9. Assertion (A): N,N-Diethylbenzene sulphonamide is insoluble in alkali. Reason
(R) Sulphonyl group attached to nitrogen atom is strong electron withdrawing group.
Ans. (b) Both Assertion (A) and Reason (R) are correct statements, but Reason (R) is not the correct explanation of the Assertion (A).
10. Assertion (A): In presence of enzyme, substrate molecule can be attacked by the reagent effectively.
Reason (R): Active sites of enzymes hold the substrate molecule in a suitable position.
Ans. (a) Both Assertion (A) and Reason (R) are correct statements, and Reason (R) is the correct explanation of the Assertion (A).
Case-Based Question
PASSAGE-1
The spontaneous flow of the solvent through a semipermeable membrane from a pure solvent to a solution or from a dilute solution to a concentrated solution is called osmosis. The phenomenon of osmosis can be demonstrated by taking two eggs of the same size. In an egg, the membrane below the shell and around the egg material is semi- permeable. The outer hard shell can be removed by putting the egg in dilute hydrochloric acid. After removing the hard shell, one egg is placed in distilled water and the other in a saturated salt solution. After some time, the egg placed in distilled water swells-up while the egg placed in salt solution shrinks. The external pressure applied to stop the osmosis is termed as osmotic pressure (a Colligative property). Reverse osmosis takes place when the applied external pressure becomes larger than the osmotic pressure.
1. What are isotonic solutions?
Ans. Solutions having equal osmotic pressure are called isotonic solutions.
2. Name one SPM which can be used in the process of reverse osmosis.
Ans. Cellulose acetate placed on a suitable support.
3. What do you expect to happen when red blood corpuscles (RBC's) are placed in 0.5% NaCl solution?
Ans. RBC's are isotonic with 0.9% NaCl solution, so they will swell and may even burst when placed in 0.5% NaCl solution.
OR
Which one of the following will have higher osmotic pressure in 1 M KCl or 1 M urea solution?
Ans. 1 M KCI will have higher osmotic pressure because its dissociates to give K+ and CI ions while urea does not dissociate into ions in the solution.
PASSAGE-2
A Lead storage battery is the most important type of secondary cell having a lead anode and a grid of lead packed with PbO2 as cathode. A 38% solution of sulphuric acid is used as electrolyte. (Density = 1.294 g mL-¹). The battery holds 3.5 L of the acid. During the discharge of the battery, the density of H2SO4 falls to 1.139 g mL. (20% H₂SO4 by mass)
1. Write the reaction taking place at the cathode when the battery is in use.
Ans.
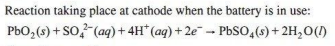
2. How much electricity in terms of Faraday is required to carry out the reduction of one mole of PbO??
Ans.

3. What is the molarity of sulphuric acid before discharge?
Ans.
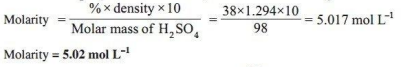
OR
(i) Lead storage battery is considered a secondary cell. Why?
Ans. (i) Because after use it can be recharged by passing current through it in the opposite direction.
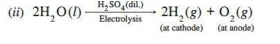
(ii) Write the products of electrolysis when dilute sulphuric acid is electrolysed using Platinum electrodes.
Ans.
PASSAGE-3
The rate of reaction is concerned with decrease in concentration of reactants or increase in the concentration of products per unit time. It can be expressed as instantaneous rate at a particular instant of time and average rate over a large interval of time. Mathematical representation of rate of reaction is give by rate law. Rate constant and order of a reaction can be determined from rate law or its integrated rate equation.
1. What is average rate of reaction?
Ans. Average rate of reaction may be defined as the change in concentration of a reactant or product in a unit time.
2. Write two factors that affect the rate of reaction.
Ans. Factors affecting rate of a chemical reaction:
(i) Concentration of reactant: Rate of reaction generally, increases with increase in the concentration of the reactants.
(ii) Temperature: Generally, rate becomes double for every 10° rise in temperature.
3. (i) What happens to rate of reaction for zero order reaction?
(ii) What is the unit of k for zero order reaction?
Ans.


Ans.
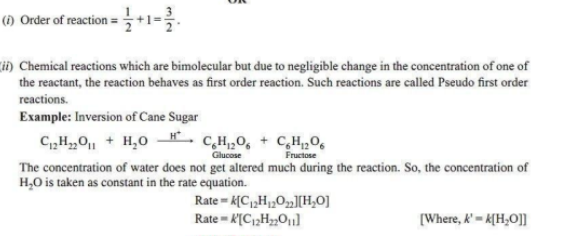
PASSAGE-4
Potassium permanganate, (KMnO4) is prepared by fusion of pyrolusite, MnO2 with KOH in the presence of an oxidising agent like KNO3. This produces the dark green potassium manganate, K2MnO4 which disproportionates in a neutral or acidic solution to give purple permanganate ion. Potassium permanganate is an important oxidising agent in acidic, alkaline as well as neutral medium.
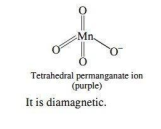
1. What is the state of hybridisation of Mn in MnO4?
Ans. sp³
2. Write an application of potassium permanganate.
Ans. It is used for the estimation of hydrogen peroxide.
3. What are the products formed after heating potassium permanganate?
Ans. K₂MnO4, O₂ and MnO, will be formed after heating of potassium permanganate.
OR
Draw the structure of permanganate ion. Is it paramagnetic or diamagnetic?
Ans.
PASSAGE- 5
The coordination compounds are of great importance. These compounds are widely present in the mineral, plant and animal worlds and are known to play many important functions in the area of analytical chemistry, metallurgy, biological systems, industry and medicine. Formation of coordination compounds is largely used in analytical chemistry for the qualitative detection and quantitative estimation of metal ions. Coordination compounds also find several important applications in the field of medicine. Several coordination compounds are also used as antidote to poisoning caused by the ingestion of poisonous metals by human beings.
1. Which complexing material is added to vegetable oils to remove the ill effects of undesired metal ions?
Ans. EDTA
2. Which complex is used in the treatment of cancer?
Ans. Cisplatin
3. How would you detect the presence of nickel in a food sample?
Ans. With the help of dimethylglyoxime which forms a red complex with Ni²* ions.
OR
What is chelate therapy?
Ans. Chelate therapy is used for the removal of excess of metal ions present in toxic amounts in the body.
PASSAGE-6
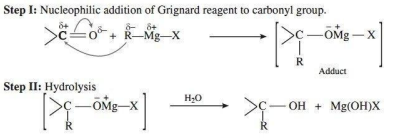
Grignard reagents are alkyl magnesium halides. Due to large electronegativity difference between carbon and magnesium, the carbon-magnesium bond has significant ionic character. The hydrocarbon part of the Grignard reagents acts as a source of carbanions. Therefore, Grignard reagents readily undergo nucleophilic addition reactions to aldehydes and ketones forming the addition products which upon hydrolysis yield alcohols.
1. What will be the product formed when chlorobenzene reacts with magnesium in presence of dry ether?
Ans. Phenyl magnesium chloride (Grignard reagent)
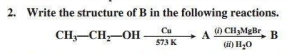
Ans.
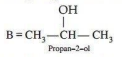
3. Give the mechanism of addition of Grignard reagent to carbonyl compound forming an alcohol.
Ans.
OR
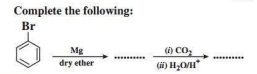
Ans.

PASSAGE-7
Alcohols and Phenols are acidic in nature. Electron withdrawing groups in phenol increase its acidic strength and electron donating groups decrease it. Alcohols undergo nucleophilic substitution with hydrogen halides to give alkyl halides. On oxidation primary alcohols yield aldehydes with mild oxidising agents and carboxylic acids with strong oxidising agents while secondary alcohols yield ketones. The presence of -OH groups in phenols activates the ring towards electrophilic substitution. Various important products are obtained from phenol like salicylaldehyde, salicylic acid, picric acid etc.
1. Give the structure of an alcohol which is resistant to oxidation?
Ans.

2. Name any one group that increases the acidic character of phenol?
Ans. 2. NO, is electron withdrawing group, due to which it increase the stability of phenoxide ion.
3. Consider the following reaction:
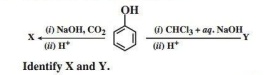
Ans.
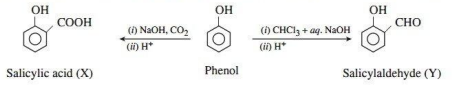
OR
p-nitrophenol is a stronger acid than phenol while p-cresol is a weaker acid. Why?
Ans. This is because-NO2 decreases electron density on oxygen of O-H group making p-nitrophenol a stronger acid. While -CH3 group increases electron density.
PASSAGE-8
Aldehydes and ketones having atleast one a-hydrogen undergo a reaction in the presence of dilute alkali as a catalyst to form ẞ-hydroxy aldehyde (aldol) or B-hydroxy ketones (ketol), respectively. The aldol and ketol readily lose water to give a, ẞ-unsaturated carbonyl compounds which are aldol condensation products and the reaction is called aldol condensation. When aldol condensation is carried out between two different aldehydes and/or ketones, it is called cross aldol condensation.
1. Give the IUPAC name of the compound formed when acetone undergoes self aldol condensation.
Ans. 4-methylpent-3-en-2-one
2. Identify the compounds that give 1, 3-diphenylprop-2-en-1-one after aldol condensation?
Ans. Benzaldehyde and acetophenone
3. Give the reaction that gives of formation of products by self aldol condensation of acetone.
Ans.
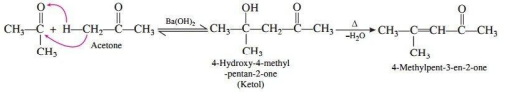
OR
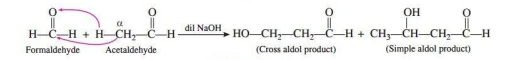
Write the equation of cross aldol condensation between formaldehyde and acetaldehyde.
Ans.
PASSAGE-9
Amines have a lone pair of electrons on nitrogen atom due to which they behave as Lewis base. Larger the value of Kb or smaller the value of pKb stronger is the base. Amines are more basic than alcohols, ethers, esters, etc. The basic character of aliphatic amines should increase with the increase of alkyl substitution. But it does not occur in a regular manner as a secondary aliphatic amine is unexpectedly more basic than a tertiary amine in solutions. Aromatic amines are weaker bases than ammonia and aliphatic amines. Electron-donating groups such as -CH3, -OCH3,-NH2, etc., increase the basicity while electron-withdrawing substituents such as-NO2, CN, halogens. etc. decrease the basicity of amines. The effect of these substituents is more at p- than at m-positions.
1. Arrange the following in increasing order of their basic strength: C₂H5NH2, CH5NH2, NH3, C6H5CH₂NH2, (C₂H₂),NH
Ans. C6H5NH2<NH3<C6H5CH2NH2<C2H5NH2 < (C2H5)2NH
2. Arrange the following compounds in increasing order of their acidic strength: Methylamine, dimethylamine, aniline, N-methylaniline
Ans. . dimethylamine < methylamine < N-methylaniline << aniline
3. (CH3)2NH is more basic than (CH3)3N in an aqueous solution. Give reason.
Ans. The basicity of amine in aqueous solution depends upon the stability of the substituted ammonium cation. Here the combination of three factors, +ve I effect of CH3 groups, hydrogen bonding and steric hindrance favour greater stability for ammonium cation of dimethyl amine than ammonium cation of trimethyl amine. Hence, dimethylamine is stronger base than trimethyl amine.
OR
Which is more acidic, aniline or ammonia?
Ans. Due to delocalization of the lone pair of electrons of the N-atom of aniline over the benzene ring, aniline is more acidic than ammonia.
PASSAGE-10
Living systems are made up of various complex biomolecule, like carbohydrates, proteins, nucleic acids, lipids, etc. Carbohydrates are optically active polyhydroxy aldehydes or ketones or molecules which provide such units on hydrolysis. They are broadly classified into three groups - monosaccharides, oligosaccharides and polysaccharides. Monosaccharides are held together by glycosidic linkages to form disaccharides like sucrose, maltose or polysaccharides like starch and cellulose. Another biomolecule: proteins are polymers of a-amino acids which are linked by peptide bonds. Ten amino acids are called essential amino acids. Structure and shape of proteins can be studied at four different levels i.e., primary, secondary, tertiary and quaternary, each level being more complex than the previous one.
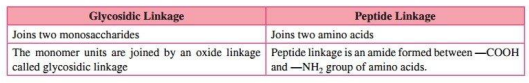
1. What is the difference between a glycosidic linkage and peptide linkage?
Ans.
2. Which amino acids are called essential amino acids?
Ans. The amino acid that cannot be synthesised in the body and must be obtained through food are known as essential amino acid. Example-Valine, Leucine, Arginine.
3. What are the common types of secondary structures of proteins? Write any two forces which-stabilise the secondary and tertiary structures of protein.
Ans. Secondary structure of the protein refers to the shape in which the polypeptide chain can exist. The shapes are due to the interaction between the atoms in the polypeptide chain. The common types of 2° proteins are:
(a) a-helix: twisted like right handed screw because of Hydrogen bonding.
(b) B-pleated sheet structure: In this all the peptide chains are stretched to maximum extent and laid side by side, which are held together by hydrogen bonds.
The two forces that stabilise the 2° and 3° structure of proteins are:
1. Hydrogen bonds
2. Disulphide linkages.
OR
Define denaturation of protein with an example. During denaturation which structures of protein lose their biological activity?
Ans. The hydrogen bonds in the native protein are disturbed or broken when the protein molecules are subjected to physical stress (like temperature change) or chemical changes like change in pH. Owing to this, the proteins lose their biological activity, which is known as denaturation of protein. Example - The egg protein undergoes coagulation when subjected to high temperature (boiling point). During denaturation only the secondary and tertiary structures are destroyed, the primary structure remains intact.
Conceptual Question
Q. 1. When and why is molality preferred over molarity in handling solutions in chemistry?
Ans. Molality is preferred in studies that involves changes in temperature as in some of the colligative properties of solutions. This is because molality depends on masses of solvent which do not change with temperature.
Q. 2. Why does a galvanic cell become dead after some time?
Ans. As the reaction proceeds, concentration of ions in anodic half keeps on increasing while in the cathodic half it keeps on decreasing. Hence, their electrode potentials also keeps on changing until they become equal and then e.m.f. of the cell becomes zero.
Q.3. Why is the probability of reaction with molecularity higher than three very rare?
Ans. The probability of more than three molecules colliding simultaneously is very small. Hence, possibility of molecularity being three is very low.
Q.4. Reactivity of transition elements decreases almost regularly from Sc to Cu. Explain.
Ans. It is due to regular increase in ionisation enthalpy.
Q. 5. Square planar complexes with coordination number of four exhibit geometrical isomerism whereas tetrahedral complexes do not. Why?
Ans. Tetrahedral complexes do not show geometrical isomerism because the relative positions of the ligands attached to the central metal atom are same with respect to each other.
Q.6. Why is t-butyl bromide more reactive towards Syl reaction as compared to n-butyl bromide?
Ans. Due to higher stability of tertiary carbocation than primary carbocation.
Q.7. Why ortho-nitrophenol is steam volalite while para-nitrophenol is not?
Ans. Due to intramolecular H-bonding o-nitrophenol exists as a discrete molecule whereas due to intermolecular H-bonding p-nitrophenol exists as associated molecules. As a result of this p-nitrophenol has higher boiling point than o-nitrophenol.
Q. 8. Write two important uses of formalin.
Ans. Formalin is used in the
(i) preservation of biological specimens.
(ii) manufacture of bakelite.
Q.9. Why do amines behave as nucleophiles?
Ans. Due to the presence of a lone pair of electrons on nitrogen atom, amines behave as nucleophiles.
Q. 10. Why are carbohydrates generally optically active?
Ans. Carbohydrates have chiral or asymmetric carbon atom.
Very Short Answer Question I
Q. 1. Explain the solubility rule "like dissolves like" in terms of intermolecular forces that exist in solutions.
Ans. A substance (solute) dissolves in a solvent if the intermolecular interactions are similar in both the components; for example, polar solutes dissolve in polar solvents and non polar solutes in non polar solvents and thus we can say "like dissolves like".
Q. 2. Give reasons:
(i) Mercury cell delivers a constant potential during its life time.
(ii) In the experimental determination of electrolytic conductance, Direct Current (DC) is not used.
Ans. (i) Overall reaction does not involve any ion whose concentration may change. Hence, it delivers a constant potential during its life time.
(ii) Direct current (DC) causes electrolysis of the solution consequently. The concentration of the electrolyte near the electrodes changes and this results in the change in the resistance (and hence the conductance) of the solution. Therefore direct current is not used.
Q. 3. What happens to the rate constant ✯ and activation energy E, as the temperature of a chemical reaction is increased? Justify.
Ans. Effect of temperature on rate of reaction: Increasing the temperature of a reaction mixture increases the fraction of molecules, which collide with energies greater than E. It is clear from the diagram alongside that with 10° rise in temperature, the area showing the fraction of molecules having energy equal to or greater than activation energy gets almost double leading to almost doubling of the rate of reaction.
Q. 4. Explain each of the following observations:
(i) Actinoids exhibit a much larger number of oxidation states than the lanthanoids.
(ii) There is hardly any increase in atomic size with increasing atomic numbers in a series of transition metals.
Ans. (i) This is due to small energy gap between 5f, 6d and 7s-subshells in actinoids.
(ii) This is because with increase in atomic number in a series, the increased nuclear charge is partly cancelled by the increased shielding effect of electrons in the d-orbitals of penultimate shell.
Q. 5. What is the relationship between observed colour of the complex and the wavelength of light absorbed by the complex?
Ans. When white light falls on the complex, some part of it is absorbed. Higher the crystal field splitting, lower will be the wavelength absorbed by the complex. The observed colour of complex is the colour generated from the wavelength left over.
Q.6. Which one of the following compounds is more easily hydrolysed by KOH and why? CH3CHCICH₂CH3 or CH3CH2CH2CI
Ans. Due to +I effect of alkyl groups, the 2º carbonium ion CH3-CH+-CH2-CH3 derived from sec. butyl chloride is more stable than the 1° carbonium ion CH3 CH2-CH₂ derived from n-propyl chloride. Therefore sec. butyl chloride gets hydrolysed more easily than n-propyl chloride under SNl conditions.
Q. 7. Which is a stronger acid-phenol or cresol? Explain.
Ans. All the cresols are weaker acids than phenols. Methyl group has +I effect (positive inductive effect) as well as hyperconjugation effect but the hyperconjugation effect predominates over the +1 effect. Since both these effects increase the electron density on the O-H bond and hence all the cresols are weaker acids than phenols. As hyperconjugation effect can operate only through ortho and para positions and not through meta positions, therefore, meta-cresol is stronger acid than ortho and para-cresols. However, due to stronger +I effect at ortho position than at para position (+I effect decreases with distance), ortho-cresol is a weaker acid than para-cresol. Thus, the order of acidic strength in increasing order is: ortho-cresol <para-cresol <meta-cresol < phenol
Q. 8. Give reasons to support the answer:
(i) Presence of Alpha hydrogen in aldehydes and ketones is essential for aldol condensation.
(ii) 3-Hydroxypentan-2-one shows positive Tollen's test.
Ans. (i) The alpha hydrogen atoms are acidic in nature due to presence of electron withdrawing carbonyl group. These can be easily removed by a base and the carbanion formed is resonance stabilized.
(ii) Tollen's reagent is a weak oxidizing agent not capable of breaking the C-C bond in ketones. Thus ketones cannot be oxidized using Tollen's reagent itself gets reduced to Ag.
Q. 9. Account for the following:
(i) pK, of aniline is more than that of methylamine.
Ans. (i) Refer to Ans. 3(i) NCERT Exercises.
(ii) Although trimethylamine and n-propylamine have the same molecular weight, but the former boils at a lower temperature (276 K) than the latter (322 K). Explain.
Ans. (ii) n-Propylamine has two H-atoms on the N-atom and hence undergoes intermolecular H-bonding, thereby raising its boiling point. Trimethylamine, (CH3)3N, being a tertiary amine does not have any H-atom on the N-atom. As a result, it does not undergo H-bonding and hence its boiling point is low.
Q. 10. Name the bases present in RNA. Which one of these is not present in DNA?
Ans. The bases present in RNA are adenine (A), guanine (G), cytosine (C) and uracil (U). Uracil is not present in DNA.
Very Short Answer Question - II
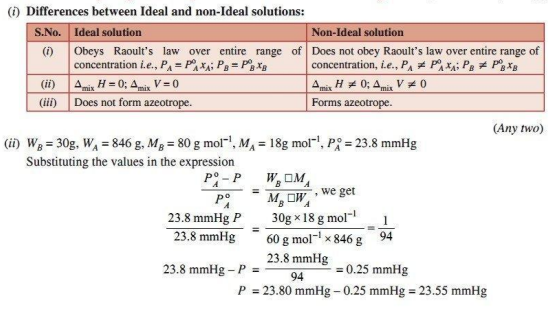
Q. 1. (i) Differentiate between Ideal solution and Non-ideal solution.
(ii) 30 g of urea is dissolved in 846 g of water. Calculate the vapour pressure of water for this solution if vapour pressure of pure water at 298 K is 23.8 mm Hg.
Ans.
Q.2. Estimate the minimum potential difference needed to reduce Al2O3 at 500°C. The free energy change for the decomposition reaction

Ans.
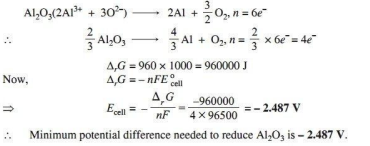
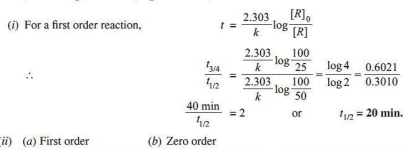
Q.3. Define half-life of a reaction. Write the expression of half-life for
(i) zero order reaction and
(ii) first order reaction.
Ans.

Q 4.
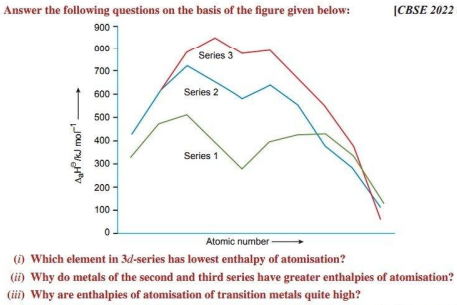
Ans. (i) In the formation of metallic bonds, no electrons from 3d-orbitals are involved in case of zinc, while in all other metals of the 3d-series, electrons from the d-orbitals are always involved in the formation of metallic bonds. That is why, the enthalpy of atomisation of zinc is the lowest in the series.
(ii) Metals of second (4d) and third series (5d) have greater enthalpy of atomisation due to larger size than that of 3d element and hence can form metal-metal bond more frequently.
(iii) The transition elements exhibit high enthalpy of atomisation because they have large number of unpaired electrons in their atoms. Due to this they have stronger interatomic interaction.
Q. 5. Write the correct formulae for the following coordination compounds:
(i) CrCl3.6H₂O (violet with 3 chloride ions precipitated as AgCl)
(ii) CrCl3.6H₂O (light green colour, with 2 chloride ions precipitated as AgCI)
(iii) CrCl3.6H₂O (dark green colour, with 1 chloride ion precipitated as AgCI)
Ans. (i) [Cr(H₂O)6]Cl,
(ii) [Cr(H2O),Cl]Cl₂.H₂O
(iii) [Cr(H₂O),Cl,]C1.2H₂O
Q.6. How do you convert the following:
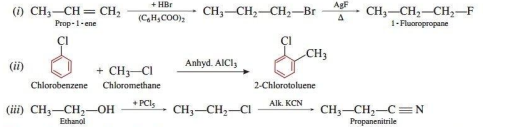
(i) Prop-1-ene to 1-fluoropropane
(ii) Chlorobenzene to 2-chlorotoluene
(iii) Ethanol to propanenitrile
Ans.
Q. 7. How do you convert the following:
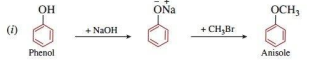
(i) Phenol to anisole
Ans.
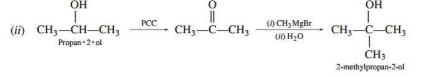
(ii) Propan-2-ol to 2-methylpropan-2-ol
Ans.
(iii) Aniline to phenol
Ans.
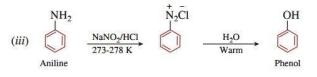
Q. 8. Give reasons:
(i) The a-hydrogen of aldehydes and ketones are acidic in nature.
Ans.

(ii) Propanone is less reactive than ethanal towards addition of HCN.
Ans. This is due to steric and electronic reasons. Sterically, the presence of two methyl groups in propanone hinders more the approach of nucleophile to carbonyl carbon than in ethanal having one methyl group. Electronically two methyl groups reduce the positivity of the carbonyl carbon more effectively in propanone than in ethanal.
(iii) Benzoic acid does not give Friedel-Crafts reaction.
Ans. (iii) Benzoic acid does not give Friedel Craft reaction because:
(a) the carboxyl group is strongly deactivating.
(b) the catalyst AICI, which is a lewis acid gets bonded to the carboxyl group strongly.
Q.9. Account for the following:
(i) Aniline cannot be prepared by the ammonolysis of chlorobenzene under normal conditions.
Ans. (i) In case of chlorobenzene, the C-Cl bond is quite difficult to break as it acquires a partial double bond character due to conjugation.
So, Under the normal conditions, ammonolysis of chlorobenzene does not yield aniline.
(ii) N-ethylethanamine boils at 329.3K and butanamine boils at 350.8K, although both are isomeric in nature.
Ans. (ii) Primary and secondary amines are engaged in intermolecular association due to hydrogen bonding between nitrogen of one and hydrogen of another molecule. Due to the presence of three hydrogen atoms, the intermolecular association is more in primary amines than in secondary amines as there are two hydrogen atoms available for hydrogen bond formation in it.
(iii) Acylation of aniline is carried out in the presence of pyridine.
Ans. (iii) During the acylation of aniline, stronger base pyridine is added. This is done in order to remove the HCI so formed during the reaction and to shift the equilibrium to the right hand side.
Q.10. (i) Give one structural difference between amylose and amylopectin
Ans. (i) Amylose is a long unbranched chain polymer of a-D(+) glucose. Amylopectin is a branched chain polymer of a-D glucose.
(ii) Name the protein and its shape present in oxygen carrier in human body.
Ans. (ii) Globular protein and its shape is spherical.
(iii) Name two fat storing tissues in human body.
Ans. (iii) Liver and adipose tissue.
Long Answer Question
Q.1.(i) Define the following terms:
(a) Azeotrope
(b) Osmotic pressure
(c) Colligative properties
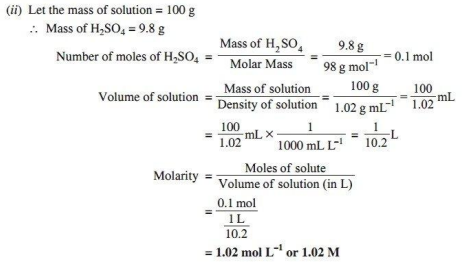
(ii) Calculate the molarity of 9.8% (w/w) solution of H₂SO4 if the density of the solution is 1.02 g mL.-1 (Molar mass of H₂SO4 = 98 g mol-1)
Ans.
(i) (a) The binary mixtures of liquids having same composition in liquid and vapour phase and boil at a constant temperature are called azeotropes.
(b) The excess of pressure which must be applied to the solution side to prevent the passage of solvent into it through a semipermeable membrane is called osmotic pressure.
(c) The properties of solutions which depend only on the number of solute particles in the solution but independent of their nature are called colligative properties.
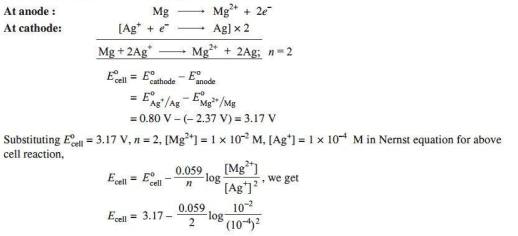
Q 2.

Ans.
Q 3.
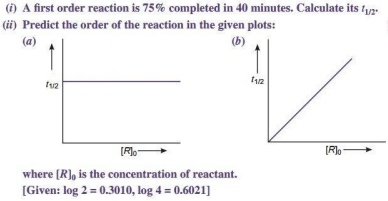
Ans.
Q 4.

Ans.

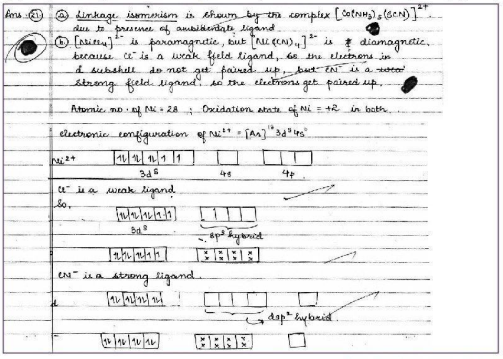
Q. 5. (i) What type of isomerism is shown by the complex [Co(NH3)5(SCN)]2+?
(ii) Why is [NICI4]-2 paramagnetic while [Ni(CN4)-2 is diamagnetic? (Atomic number of Ni = 28)
(iii) Why are low spin tetrahedral complexes rarely observed?
Ans.

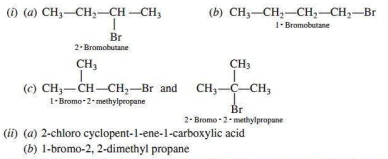
Q. 6. (1) Among all the isomers of molecular formula C4H9Br, identify
(a) the one isomer which is optically active.
(b) the one isomer which is highly reactive towards SN2.
(c) the two isomers which give same product on dehydrohalogenation with alcoholic KOH.
(ii) Give IUPAC name of the following organic compounds:

Ans.
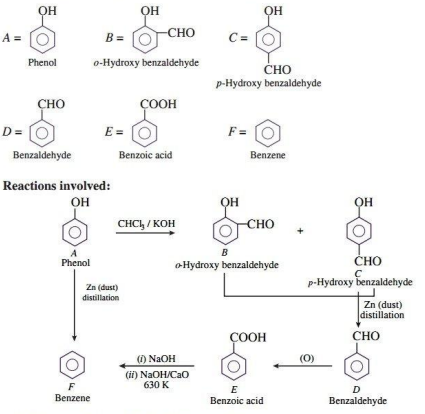
Q.7. An aromatic compound 'A' on treatment with CHCl3/KOH gives two compounds 'B' and 'C'. Both B and C give the same product 'D' when distilled with zinc dust. Oxidation of D gives E having molecular formula C7H6O₂. The sodium salt of E on heating with sodalime gives F which may also be obtained by distilling A with zinc dust. Identify A to F.
Ans.
Q.8. Write the structures of A, B, C, D and E in the following reactions:

Ans.
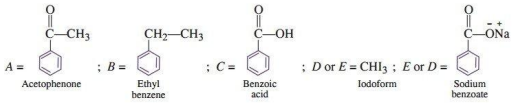
Q.9. An aromatic compound 'A' of molecular formula C7H6O2 undergoes a series of reactions as shown below. Write the structures of A, B, C, D and E in the following reactions:

Ans.
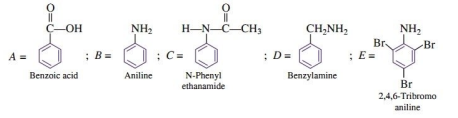
Q. 10. (i) (a) What is the difference between native protein and denatured protein?
Ans. (a) Protein found in a biological system with unique three dimensional structure and biological activity is called native protein.
When a protein in its native form is subjected to change such as change in temperature, change in pH, its 2° and 3° structures are destroyed and it loses its biological activity. The protein thus formed is called denatured protein.
(b) Which one of the following is a disaccharide?
Glucose, Lactose, Amylose, Fructose
Ans. (b) Lactose
(c) Write the name of the vitamin responsible for the coagulation of blood.
Ans. (c) Vitamin K.
(ii) Define the following terms:
(a) Native protein
Ans. (a) Protein found in biological system with unique three dimensional structure and biological activity is called native protein.
(b) Nucleotide
Ans. (b) A unit formed by the combination of nitrogenous base, pentose sugar and phosphate.
-
Read Also -
CBSE 12 Chemistry Exam 2024 : Chapter (Solutions) DPP (Daily Practice Paper) with Detailed Solution
Read Also -
CBSE Physics 12th Exam 2024 : Most Important Chapter-Wise Short Answer Type Questions; Download PDF
CBSE Biology 12th Exam 2024 : Most Important Chapter-Wise Short Answer Type Questions; Download PDF







 Profile
Profile Signout
Signout








 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










