The Central Board of Secondary Education (CBSE) conducted the Class 12 Chemistry board exam today, February 27, 2024. The exam was conducted between 10.30 am to 1.30 pm.
Now, as the exam is over, we bring you post-exam review, paper analysis and student feedback on the difficulty level of CBSE Class 12 Chemistry exam 2023. Compare your answers with the CBSE Class 12 Chemistry Question Paper 2024 Solved PDF available on this page.
👉 CBSE Class 12 Chemistry Question Paper 2024 with Answer Key
CBSE Class 12 Chemistry Paper Review 2024
CBSE Class 12 Chemistry Exam 2024 is reviewed to be of moderate difficulty level. Students have mentioned that the paper pattern was similar to the CBSE sample paper and no question in the paper was out of syllabus.
Note* The exam analysis shared here is entirely based on the responses and reactions shared by students and teachers. The perspectives may vary from one individual to another, so the analysis of the CBSE Class 12 Chemistry Exam 2024 must be accepted as a subjective evaluation. It should not affect your perception in any way.
Type of Questions Asked in CBSE Class 12 Chemistry Exam 2024
The CBSE Class 12 Chemistry question paper was for 80 marks and had 33 questions. Students were allowed to complete the paper within 3 hours.
The paper was divided into five sections:
|
Section A |
Q. No. 1-16: MCQs |
1 mark each |
|
Section B |
Q. No. 17-21 |
2 marks each |
|
Section C |
Q. No. 22-28 |
3 marks each |
|
Section D |
Q. No. 29-30 (Case-based) |
4 marks each |
|
Section E |
Q. No. 31-33 |
5 marks each |
All questions were compulsory. However, internal choices were provided in some questions.
CBSE Class 12 Chemistry Exam Analysis 2024: Students’ Reactions
As per the general reviews, students found the paper moderate and balanced. They said the paper was NCERT-based, and covered all relevant topics.
Some of the general reactions include:
- MCQ part of the paper was slightly tricky but manageable.
- Section B was easy.
- Questions in section C were direct.
- Case-based questions in section D were moderate.
- Long answer type questions in the paper were direct.
CBSE Class 12 Chemistry Paper Analysis 2024: Experts' Review
Experts and educators have mentioned that the chemistry paper was balanced and had a moderate difficulty level. Most of the questions were direct and based on NCERT. The paper pattern followed the sample paper provided by CBSE. The majority of the questions in the paper ranged from easy to moderate, except for a few that could have posed a challenge for students. Overall, the CBSE Class 12 Chemistry paper was quite average for almost all the students
CBSE Chemistry Answer Key Class 10 2024 - (Set-56/4/1/2/1)
SECTION-A
Question No. 1 to 16 are Multiple Choice type questions carrying 1 mark each.
1. The molar ionic conductivities of Ca2+ and Cl- are 119.0 and 76.3 S cm² mol-1 respectively. The value of limiting molar conductivity of CaCl, will be:
(A) 195.3 S cm² mol-1
(C) 314.3 S cm² mol-1
(B) 43.3 S cm² mol-1
(D) 271.6 S cm² mol-1
2. Consider the following reaction:
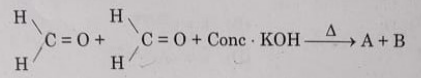
Identify A and B from the given options:
(A) A-Methanol, B-Potassium formate
(B) A-Ethanol, B- Potassium formate
(C) A-Methanal, B - Ethanol
(D) A-Methanol, B - Potassium acetate
3. Which of the following acids represents Vitamin C ?
(A) Saccharic acid
(B) Gluconic acid
(C) Ascorbic acid
(D) Benzoic acid
4. Rosenmund reduction is used for the preparation of Aldehydes. The catalyst used in this reaction is
(A) Pd - BaSO4
(B) Anhydrous AlCl3
(C) Iron (III) oxide
(D) HgSO4
5. Which alkyl halide from the given options will undergo SNl reaction faster?
(A) (CH3)3C-Br
(B) (CH3)2CH-Br
(C) CH3-CH2-Br
(D) (CH3)3C-CH-Br
6. From the elements of 3d series given below, which element shows the maximum number of oxidation states?
(A) Scandium
(B) Manganese
(C) Chromium
(D) Titanium
7. The correct Mathematical expression of Arrhenius equation is

Ans : C
8. Identify the tertiary amine from the following:
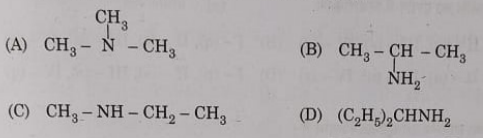
Ans : D
9. Nucleophilic addition of Grignard reagent to ketones followed by hydrolysis with dilute acids forms:
(A) Alkene
(B) Primary alcohol.
(C) Tertiary alcohol
(D) Secondary alcohol
Ans : C
10. In a given graph of zero order reaction, the slope and intercept are:
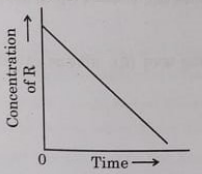
(A) Slope = k, Intercept = [R]o
(B) Slope =-k, Intercept = [R]o
(C) Slope = k/2.303, Intercept = In[R]o
(D) Slope = -k/2.303, Intercept = In Ax
Ans B
11. Match the reagents required for the given reactions:

(A) I-(r), II - (p), III-(s), IV - (q)
(B) I-(q), II (r), III - (p), IV - (s)
(C) I-(s), II (q), III - (p), IV - (r)
(D) I-(p), II - (s), III - (r), IV - (q)
Ans : A
12. The general electronic configuration of d-block elements is :
(A) (n-1) d1-10ns1-2
(B) (n-1) d10ns1-2
(C) (n-1) d10ns2-3
(D) (n-1) d0ns1-2
For questions number 13 to 16, two statements are given - one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (A), (B), (C) and (D) as given below:
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
13. Assertion (A): p-nitrophenol is less acidic than phenol.
Reason (R): Nitro group is electron withdrawing and helps in the stabilisation of p-nitrophenoxide ion.
Ans: The statement and the reason are both incorrect. Here's the corrected analysis:
14. Assertion (A): Benzoic acid does not undergo Friedel - Crafts reaction.
Reason (R): Carboxyl group is deactivating and the catalyst aluminium chloride gets bonded to the carboxyl group.
Ans: Both Assertion (A) and Reason (R) are true, but Reason (R) is not the only reason for Assertion (A).
15. Assertion (A): Fructose is a reducing sugar.
Reason (R): Fructose does not reduce Fehling solution and Tollen's reagent.
Ans: Assertion (A) is true, but Reason (R) is false.
16.

Ans: Both the assertion and the reason are correct.
SECTION - B
17. Define the following terms:
(a) Order of a reaction
(b) Activation energy
Ans: (i) Order of a reaction: It may be defined as the sum of exponents of the concentration terms in the rate law expression.
(ii) Activation energy: It may be defined as the extra amount of energy over and above the average energy of reactants which must be supplied to them to undergo a chemical reaction.
18. 18g of a non-volatile solute is dissolved in 200 g of H2O freezes at 272.07 K. Calculate the molecular mass of solute (Kf for water = 1.86 K kg mol-1)
Ans:
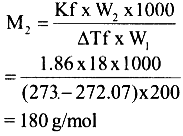
A) Which compound in the given pair would undergo SN2 reaction at a faster rate and why?
CH3-CH2-I and CH3-CH2- Br
Ans: Primary alkyl halides prefer to undergo SN2 reactions than tertiary alkyl halides because of less steric hindrance experienced by the approaching nucleophile. Hence, out of the given pair (CH3−CH2−Br) would undergo SN2 reaction faster.
(B) Arrange the following compounds in the increasing order of their boiling points:
Butane, 1-Bromobutane, 1-Iodobutane, 1-Chlorobutane
Ans: Butane < 1-Chlorobutane < 1-Bromobutane < 1-Iodobutane
20. (a) Write the stepwise mechanism of nucleophilic addition reactions in the carbonyl compounds.
OR
(b) How will you convert the following:
(i) Toluene to benzoic acid.
(ii) Ethanol to 3-Hydroxybutanal
21. (a) What happens when glucose reacts with bromine water? Write chemical equation.
(b) Two bases are mentioned below, identify which is present in DNA
and which one is present in RNA:
(i) Thymine, (ii) Uracil.







 Profile
Profile Signout
Signout








 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










