The CBSE Class 12 Chemistry Paper 2025 exam was conducted today, February 27, 2025. The exam lasted for 3 hours, conducted from 10:30 am to 1:30 pm, with an additional 15 minutes given for reading the question paper.
Here we are providing a detailed analysis of student responses, expert opinions and the difficulty level of the paper including expected good scores, time-consuming section and longest question.
We have shared the CBSE Class 12 Chemistry Question Paper 2025 PDF and answer key to help students check their answers and evaluate their performance. Direct link to download CBSE 12th Chemistry Question Paper 2025 to be avialble on this page.
👉 CBSE Class 12 Chemistry Question Paper 2025 with Answer Key (Set - 1)
👉 CBSE Class 12 Chemistry Question Paper 2025 with Answer Key (Set - 2)
👉 CBSE Class 12 Chemistry Question Paper 2025 with Answer Key (Set - 3)
CBSE Class 12 Chemistry Paper 2025: Pattern
The CBSE Class 12 chemistry question paper pattern 2025 is given below.
| Section | Question type | Number of questions | Marks per question | Total marks |
| Section A | Multiple Choice Questions (MCQ) | 16 | 1 | 16 |
| Section B | Very short answer questions | 5 | 2 | 10 |
| Section C | Short answer questions | 7 | 3 | 21 |
| Section D | Case-based questions | 2 | 4 | 8 |
| Section E | Long answer questions | 3 | 5 | 15 |
CBSE 12th Chemistry Student Reviews 2025
The details of reviews given by students on CBSE 12th Chemistry paper are updated here:
- "The paper was moderately difficult. Organic chemistry had direct questions, but some numerical in physical chemistry were tricky."
- "Many questions were from NCERT, especially in the inorganic section. However, some application-based questions required extra thinking."
- "There were many NCERT-based direct questions. Assertion-reason questions were conceptual but not too tough."
CBSE Class 12 Chemistry Exam 2025: Difficulty Level & Student Reactions
Difficulty Level
Based on initial feedback, the CBSE Class 12 Chemistry exam 2025 was of moderate difficulty. The paper included a balanced mix of conceptual, numerical, and application-based questions. While most students found the organic chemistry section easier, some reported that numerical questions from physical chemistry were tricky and required in-depth understanding.
CBSE 12th Chemistry Exam Analysis 2025
Find the complete CBSE 12th Chemistry exam analysis 2025 in the following table.
| Parameter | CBSE 12th Chemistry Exam Analysis 2025 |
| Overall difficulty level of the paper | Moderate to Tough |
| Difficulty level of Section A | Moderate |
| Difficulty level of Section B | Tough |
| Difficulty level of Section C | Moderate |
| Difficulty level of Section D | Moderate |
| Difficulty level of Section E | Moderate to Tough |
| Overall expected good score | 55+ |
| Was the overall paper time-consuming? | Assertion-reasoning and case-based questions |
| Which section was the lengthiest? | Physical Chemistry |
CBSE Class 12 Chemistry Exam Analysis And Feedback
Now that the CBSE Class 12 Chemistry exam 2025 is over, subject experts and educators are analysing the question paper. The exam was structured to test students' knowledge of chemical concepts, numerical problem-solving skills, and application of theories.
The question paper covered important topics such as:
- Solid State and Solutions
- Electrochemistry and Chemical Kinetics
- Coordination Compounds
- Organic Chemistry – Haloalkanes, Alcohols, Phenols, and Aldehydes
- Biomolecules and Polymers
- p-Block, d-Block, and f-Block Elements
- Chemistry in Everyday Life
Experts noted that the paper followed the CBSE syllabus and sample paper pattern, making it fair and predictable for students who prepared well.
Student and Teacher Reactions
Students' Reactions
Mixed reactions were seen among students after the exam:
- Many students found the organic chemistry section straightforward, especially those who practised NCERT questions.
- Numerical questions from physical chemistry were slightly challenging and required step-by-step solving.
- Assertion-reasoning and case-based questions tested conceptual clarity and critical thinking.
- Time management was crucial, as some students felt the paper was lengthy.
Teachers' Reactions
Educators appreciated the exam pattern, highlighting that:
- The paper was well-balanced and aligned with the CBSE syllabus.
- Case-based and assertion-reasoning questions required conceptual understanding rather than rote learning.
- Students who focused on NCERT textbooks and previous years' papers would find the paper manageable.
Teachers believe that students with a solid understanding of concepts and consistent practice of numerical questions can score well in this exam.
CBSE Class 12th Chemistry Answer Key 2025
The CBSE Class 12 Chemistry exam 2025 is over, and students are eagerly waiting for the answer key. The unofficial CBSE 12th Chemistry answer key will be available to help students analyze their performance.
SECTION - A
16 × 1 = 16
Questions No. 1 to 16 are Multiple Choice type questions carrying 1 mark each.
1. The charge required for the reduction of 1 mol of MnO4−to MnO2 is
(A) 1 F
(B) 3 F
(C) 5 F
(D) 6 F
Correct Answer: (B) 3 F
2. Which among the following is a false statement?
(A) Rate of zero-order reaction is independent of the initial concentration of the reactant.
(B) Half-life of a zero-order reaction is inversely proportional to the rate constant.
(C) Molecularity of a reaction may be zero.
(D) For a first-order reaction, t1/2= 0.693/k.
Correct Answer: (C) Molecularity of a reaction may be zero.
3. The number of molecules that react with each other in an elementary reaction is a measure of the
(A) the activation energy of the reaction
(B) stoichiometry of the reaction
(C) the molecularity of the reaction
(D) order of the reaction
Correct Answer: (C) molecularity of the reaction
4. The element having [Ar]3d104s2 electronic configuration is
(A) Cu
(B) Zn
(C) Cr
(D) Mn
Correct Answer: (B) Zn
5. The complex ions [Co(NH3)5(NO2)]2+and [Co(NH3)5(ONO)]2+ are called
(A) Ionization isomers
(B) Linkage isomers
(C) Co-ordination isomers
(D) Geometrical isomers
Correct Answer: (B) Linkage isomers
6. The diamagnetic species is:
(A) [Ni(CN)4]2-
(B) [NiCl4]2−
(C) [Fe(CN)6]3−
(D) [CoF6]3−
Correct Answer: (A) [Ni(CN)4]2-
7. Which is the correct IUPAC name for the given compound?
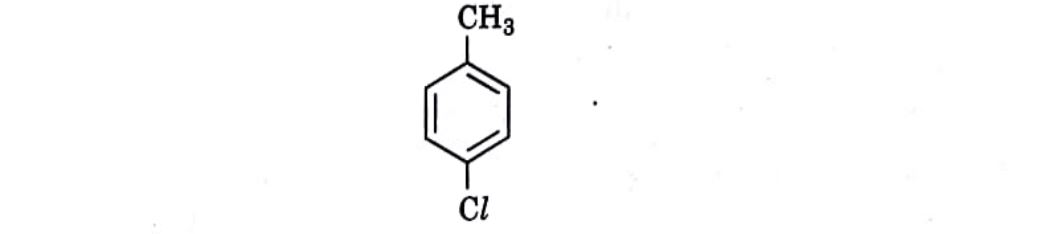
(A) Methylchlorobenzene
(B) Toluene
(C) 1-Chloro-4-Methylbenzene
(D) 1-Methyl-4-Chlorobenzene
Correct Answer: (C) 1-Chloro-4-Methylbenzene
8. What will be formed after the oxidation reaction of secondary alcohol with chromic anhydride (CrO3)?
(A) Aldehyde
(B) Ketone
(C) Carboxylic acid
(D) Ester
Correct Answer: (B) Ketone
9. The conversion of phenol to salicylic acid can be accomplished by
(A) Reimer-Tiemann reaction
(B) Friedel-Crafts reaction
(C) Kolbe reaction
(D) Coupling reaction
Correct Answer: (A) Reimer-Tiemann reaction
10. Which of the following is/are examples of denaturation of protein?
(A) Coagulation of egg white
(B) Curdling of milk
(C) Clotting of blood
(D) Both (A) and (B)
Correct Answer: (D) Both (A) and (B)
11. Nucleotides are joined together by
(A) Glycosidic linkage
(B) Peptide linkage
(C) Hydrogen bonding
(D) Phosphodiester linkage
Correct Answer: (D) Phosphodiester linkage
12. Scurvy is caused due to deficiency of
(A) Vitamin B1
(B) Vitamin B2
(C) Ascorbic acid
(D) Glutamic acid
Correct Answer: (C) Ascorbic acid
For questions numbers 13 to 16, two statements are given – one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (A), (B), (C), and (D) as given below:
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
13. Assertion (A): In a first-order reaction, if the concentration of the reactant is doubled, its half-life is also doubled.
Reason (R): The half-life of a reaction does not depend upon the initial concentration of the reactant in a first-order reaction.
Correct Answer: (D) Assertion (A) is false, but Reason (R) is true.
14. Assertion (A): Cu cannot liberate H2 in reaction with dilute mineral acids.
Reason (R): Cu has a positive electrode potential.
Correct Answer: (A) Both Assertion (A) and Reason (R) are true, and Reason (R) is the correct explanation of the Assertion (A).
15. Assertion (A): Aromatic primary amines cannot be prepared by Gabriel Phthalimide synthesis.
Reason (R): Aryl halides do not undergo nucleophilic substitution reaction with the anion formed by phthalimide.
Correct Answer: (A) Both Assertion (A) and Reason (R) are true, and Reason (R) is the correct explanation of the Assertion (A).
16. Assertion (A): Vitamin D cannot be stored in our body.
Reason (R): Vitamin D is a fat-soluble vitamin and is not excreted from the body in urine.
Correct Answer: (D) Assertion (A) is false, but Reason (R) is true.
We are adding some more answers. Keep refreshing the article.







 Profile
Profile Signout
Signout








 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










