The CBSE Class 12 pre-board exams for 2024-25 are an important step in your exam preparation. These exams help you check how well you’re prepared for the final board exams.
To help you study better, we’ve created a list of the most important Chemistry questions with answers. This Chemistry questions focuses on essential topics to help you perform well.
The CBSE Pre-board Exam Chemistry questions cover key topics. They include objective, short/long answer, and competency-based questions, all matching the latest exam pattern.
CBSE 12 Pre-board Exam 2024-25 Chemistry Most Important Questions
1. Which one of the following first row transition elements is expected to have the highest third ionization enthalpy ?
(A) Iron (Z = 26)
(B) Manganese (Z = 25)
(C) Chromium (Z = 24)
(D) Vanadium (Z = 23)
Ans. (B) Manganese (Z = 25)
2. Which of the following compounds will give a ketone on oxidation with chromic anhydride (CrO3) ?
(A) (CH3)2CH — CH2OH
(B) CH3CH2CH2OH
(C) (CH3)3C — OH
(D) CH3 — CH2 — CH — CH3
|
OH
Ans. (D) CH3 — CH2 — CH — CH3
|
OH
3. Two among the three components of DNA are β-D-2-deoxyribose and a heterocyclic base. The third component is :
(A) Adenine
(B) Phosphoric acid
(C) Sulphuric acid
(D) Uracil
Ans. (B) Phosphoric acid
4. For an electrolyte undergoing association in a solvent, the van't Hoff factor:
(A) is always greater than one
(B) has negative value
(C) has zero value
(D) is always less than one
Ans. (D) is always less than one
| Download PDF | |
| CBSE 12 Pre-board Exam 2024-25 Chemistry Most Important Questions | Click Here |
5. For the reaction X + 2Y → P, the differential form equation of the rate law is :
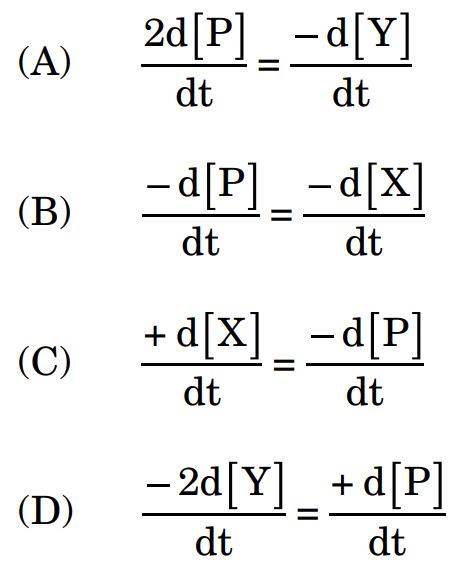
Ans.
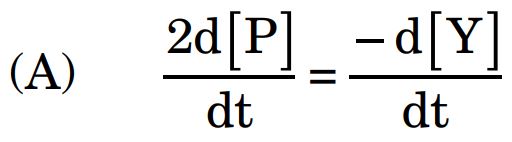
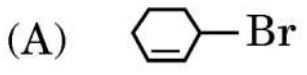
6. The compound which undergoes SN1 reaction most rapidly is :
Ans.
7. Acetic acid reacts with PCl5 to give :
(A) Cl — CH2 — COCl
(B) Cl — CH2 — COOH
(C) CH3 — COCl
(D) CCl3 — COOH
Ans. (C) CH3 — COCl
8. The formation of cyanohydrin from an aldehyde is an example of :
(A) nucleophilic addition
(B) electrophilic addition
(C) nucleophilic substitution
(D) electrophilic substitution
Ans. (A) nucleophilic addition
9. In the Arrhenius equation, when log k is plotted against 1/T, a straight line is obtained whose :
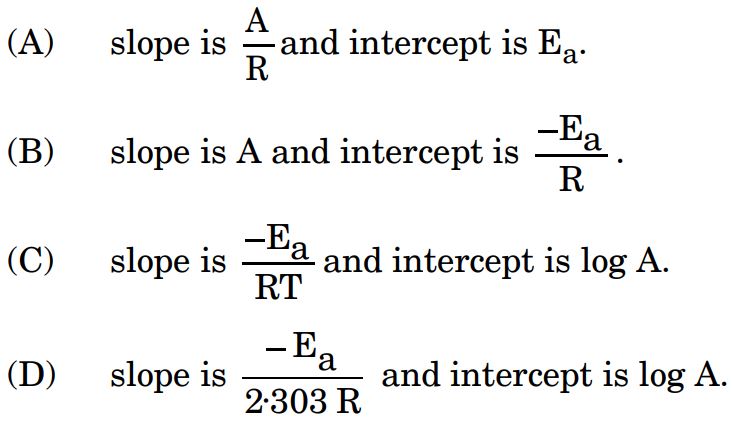
Ans.
10. The reaction of an alkyl halide with sodium alkoxide forming ether is known as :
(A) Wurtz reaction
(B) Reimer-Tiemann reaction
(C) Williamson synthesis
(D) Kolbe reaction
Ans. (C) Williamson synthesis
11. The correct order of the ease of dehydration of the following alcohols by the action of conc. H2SO4 is :
(A) (CH3)3C — OH > (CH3)2CH — OH > CH3CH2 — OH
(B) (CH3)2CH — OH > CH3CH2 — OH > (CH3)3C — OH
(C) CH3CH2 — OH > (CH3)2CH — OH > (CH3)3C — OH
(D) (CH3)2CH — OH > (CH3)3C — OH > CH3CH2 — OH
Ans. (A) (CH3)3C — OH > (CH3)2CH — OH > CH3CH2 — OH
12. Which functional groups of glucose interact to form cyclic hemiacetal leading to pyranose structure ?
(A) Aldehyde group and hydroxyl group at C - 4
(B) Aldehyde group and hydroxyl group at C - 5
(C) Ketone group and hydroxyl group at C - 4
(D) Ketone group and hydroxyl group at C - 5
Ans. (B) Aldehyde group and hydroxyl group at C - 5
For Questions number 13 to 16, two statements are given — one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (A), (B), (C) and (D) as given below.
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is notthe correct explanation of the Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
13. Assertion (A) : When NaCl is added to water a depression in freezing point is observed.
Reason (R) : NaCl undergoes dissociation in water.
Ans. (B) Both Assertion (A) and Reason (R) are true, but Reason (R) is notthe correct explanation of the Assertion (A).
14. Assertion (A) : Separation of Zr and Hf is difficult.
Reason (R) : Zr and Hf have similar radii due to lanthanoid contraction.
Ans. (A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
15. Assertion (A) : The pKa of ethanoic acid is lower than that of Cl - CH2 - COOH.
Reason (R) : Chlorine shows electron withdrawing (I) effect which increases the acidic character of Cl - CH2 - COOH.
Ans. (D) Assertion (A) is false, but Reason (R) is true.
16. Assertion (A) : Aniline is a stronger base than ammonia.
Reason (R) : The unshared electron pair on nitrogen atom in aniline becomes less available for protonation due to resonance.
Ans. (D) Assertion (A) is false, but Reason (R) is true.
17. Calculate the potential of Iron electrode in which the concentration of Fe2+ ion is 0·01 M.
(EoFe2+/Fe = 0·45 V at 298 K)
[Given : log 10 = 1]
Ans.
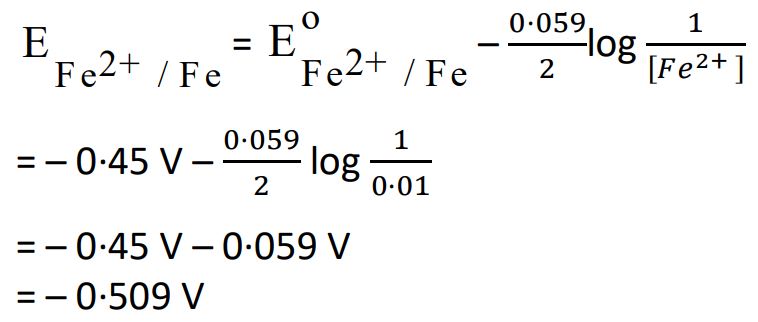
18. Define molecularity of the reaction. State any one condition in which a bimolecular reaction may be kinetically of first order.
(a) HI
(b) Conc. HNO3
Ans. The number of reacting species taking part in an elementary reaction, which must collide simultaneously in order to bring about a chemical reaction is called molecularity of a reaction.
When one of the reactants is in excess.
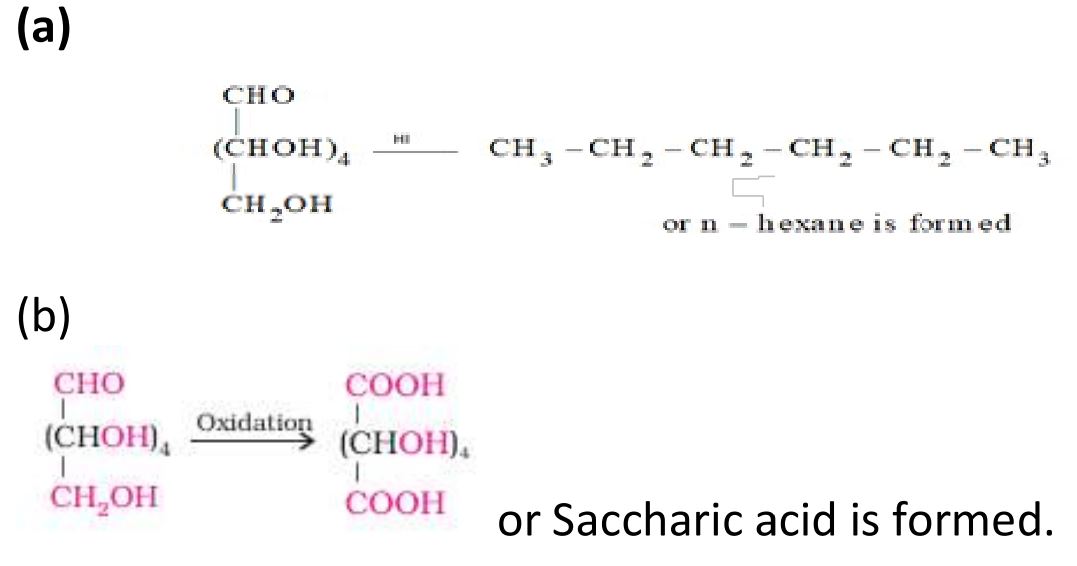
19. What happens when D-glucose is treated with the following reagents ?
Ans.
20. (a) Draw the structures of major monohalo products in each of the following reactions :
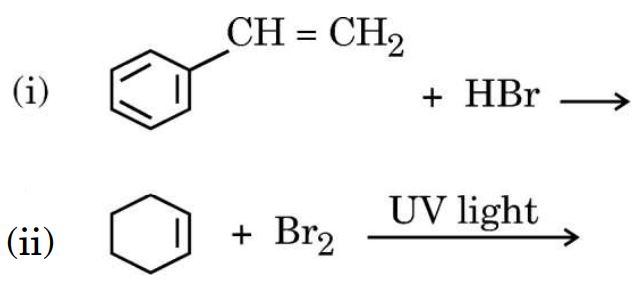
Ans.
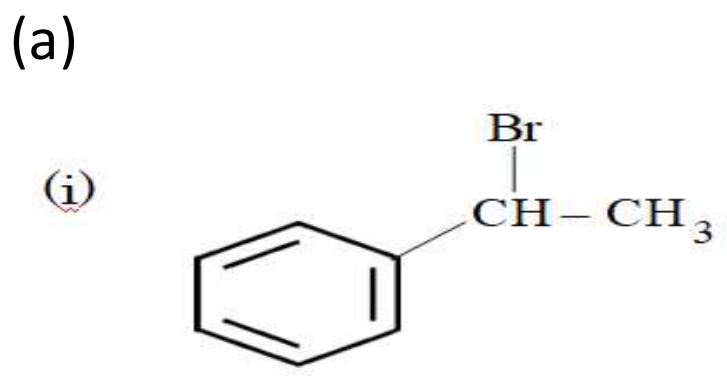
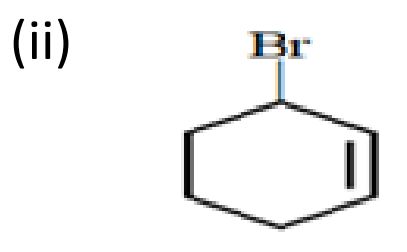
OR
(b) Give reasons for the following :
(i) Grignard reagent should be prepared under anhydrous conditions.
Ans. It reacts with water to form alkane.
(ii) Alkyl halides give alcohol with aqueous KOH whereas in the presence of alcoholic KOH, alkenes are formed.
Ans. Alcoholic KOH acts as a stronger base than aqueous KOH leads to elimination reaction of alkyl halide. / alkoxide ions in alcoholic KOH acts as a stronger base due to which elimination reaction takes place.
21. Write the chemical equation when :
(a) Butan-2-one is treated with Zn(Hg) and conc. HCl.
(b) Two molecules of benzaldehyde are treated with conc. NaOH.
Ans.
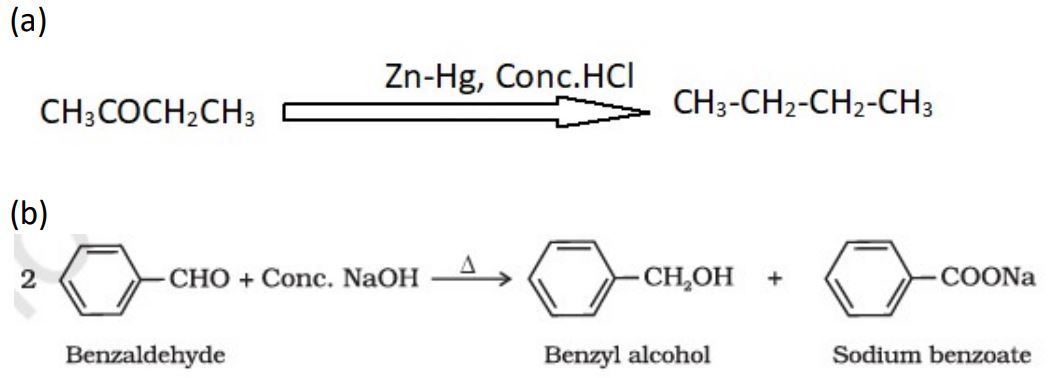
22. When a certain conductivity cell was filled with 0·05 M KCl solution, it has a resistance of 100 ohm at 25ºC. When the same cell was filled with 0·02 M AgNO3 solution, the resistance was 90 ohm. Calculate the conductivity and molar conductivity of AgNO3 solution.
(Given : Conductivity of 0·05 M KCl solution = 1·35 10-2 ohm-1cm-1)
Ans.
Cell constant(G*) = Conductivity x Resistance
= 1.35 x 10-2 x 100
= 1.35 cm-1
Cell constant (G*) = Conductivity x Resistance
1.35cm-1k x 90
1.35/90 = k
k = 0.015 Scm-1
23. The following initial rate data were obtained for the reaction :
2NO (g) + Br2 (g) ⟶ 2NOBr (g)
| Expt. No. | [NO]/mol L-1 | [Br2]/mol L-1 | Initial Rate (mol L-1s-1) |
| 1 | 0·05 | 0·05 | 1·0 x 10-3 |
| 2 | 0·05 | 0·15 | 3·0 x 10-3 |
| 3 | 0·15 | 0·05 | 9·0 x 10-3 |
(a) What is the order with respect to NO and Br2 in the reaction ?
(b) Calculate the rate constant (k).
(c) Determine the rate of reaction when concentration of NO and Br2 are 0·4 M and 0·2 M, respectively.
Ans.
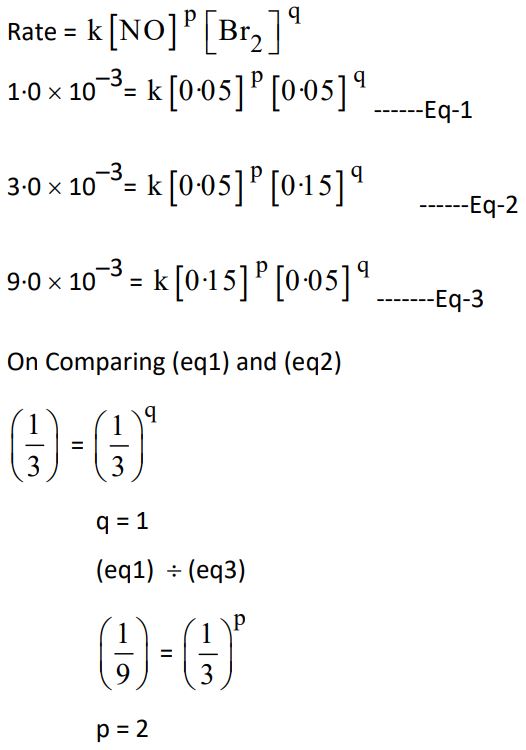

24. (a) Write the formula for the following coordination compound :
Potassium tetrahydroxidozincate (II)
Ans. K2[Zn(OH)4]
(b) Arrange the following complexes in the increasing order of conductivity of their solution :
[Cr(NH3)5Cl]Cl2, [Cr(NH3)3Cl3], [Cr(NH3)6]Cl3
Ans. [Cr (NH3)3Cl3] < [Cr(NH3)5Cl]Cl2 < [Cr(NH3)6]Cl3
(c) Identify the type of isomerism exhibited by the following complexes :
(i) [Co(NH3)5NO2]2+
(ii) [Co(en)3]Cl3
Ans. (i) Linkage isomerism
(ii) Optical isomerism
25. (a) Which of the following is an allylic halide ?
(i) CH3 — CH = CH — Br
(ii) CH2 = CH — CH — CH3
|
Br
Ans. CH2 = CH — CH — CH3
|
Br
(b) Out of chlorobenzene and 2,4,6-trinitrochlorobenzene, which is more reactive towards nucleophilic substitution and why ?
Ans. 2,4,6-trinitrochlorobenzene, because of electron withdrawing nature of –NO2 group.
(c) Which isomer of C4H9Cl has the lowest boiling point ?
Ans. (CH3)3C-CI / tert-butyl chloride
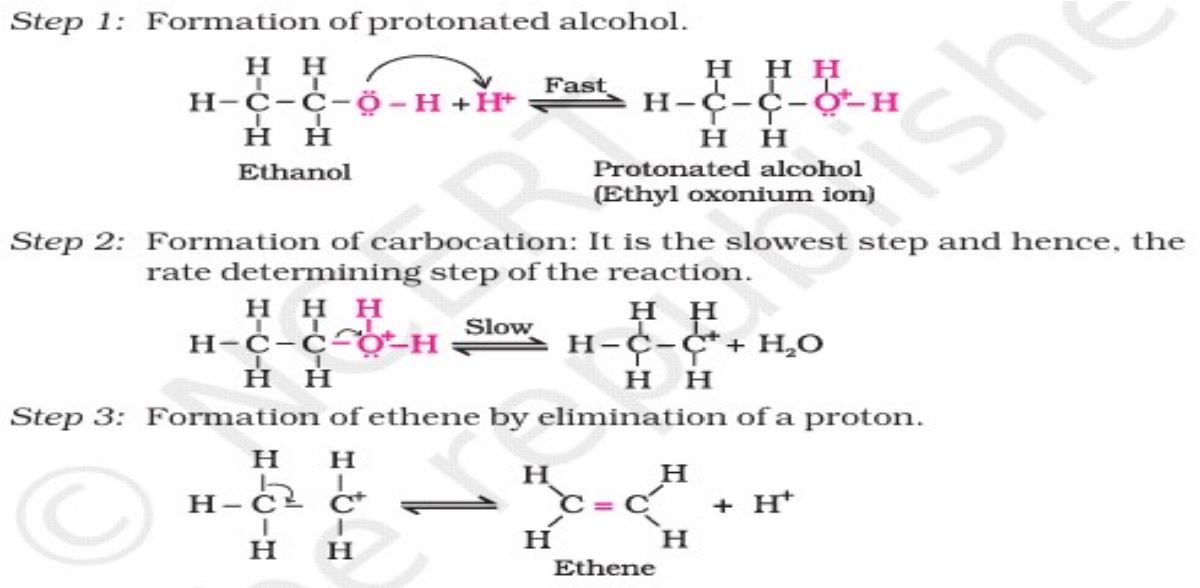
26. (a) Write the mechanism of the following reaction :
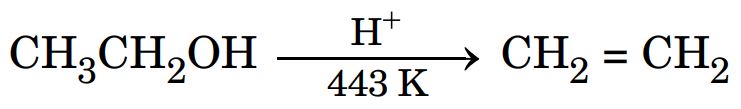
Ans.
(b) Write the main product in each of the following reactions :
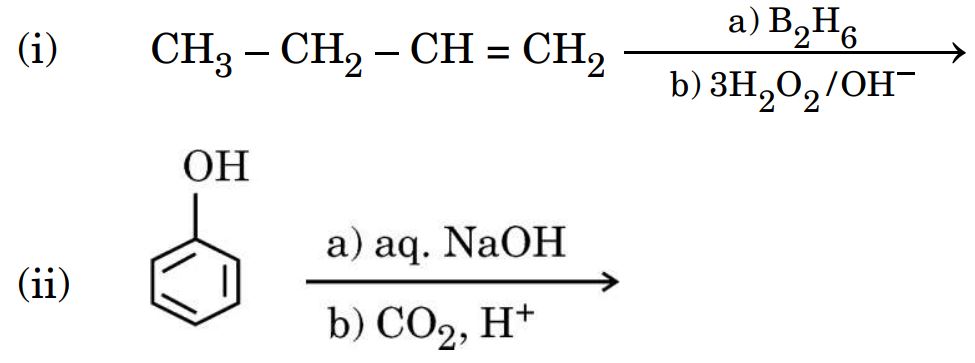
Ans. (i) CH3 – CH2 – CH2 – CH2 – OH
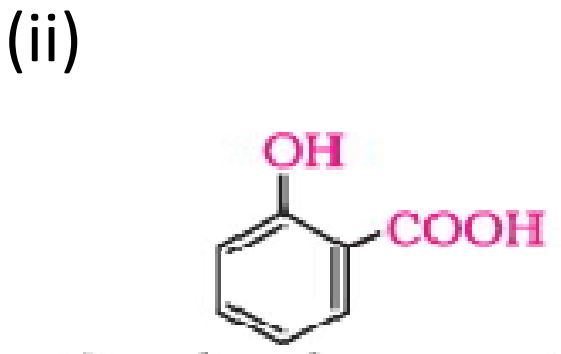
27. Answer the following : (any three)
(a) What is peptide linkage ?
Ans. A linkage which joins two amino acids through -CONH-bond.
(b) What type of bonds hold a DNA double helix together ?
Ans. Hydrogen bonding
(c) Which one of the following is a polysaccharide ?
Sucrose, Glucose, Starch, Fructose
Ans. Starch
(d) Give one example each for water-soluble vitamins and fat-soluble vitamins.
Ans. Water soluble - Vitamin B/C
Fat soluble - A, D, E, K (Any one)
28. Compound (A) (C6H12O2) on reduction with LiAlH4 gives two compounds (B) and (C). The compound (B) on oxidation with PCC gives compound (D) which upon treatment with dilute NaOH and subsequent heating gives compound (E). Compound (E) on catalytic hydrogenation gives compound (C). The compound (D) is oxidized further to give compound (F) which is found to be a monobasic acid (Molecular weight = 60). Identify the compounds (A), (B), (C), (D), (E) and (F).
Ans.
(A) → CH3 CH2 CH2 COO CH2 CH3 / CH3 COOCH2CH2 CH2 CH3
(B) → CH3 CH2 OH
(C) → CH3 CH2 CH2 CH2 OH
(D) → CH3 CHO
(E) → CH3 – CH = CH— CHO
(F) → CH3COOH (Either structure or name of A to F)
The following questions are case-based questions. Read the case carefully and answer the questions that follow.
29. Batteries and fuel cells are very useful forms of galvanic cell. Any battery or cell that we use as a source of electrical energy is basically a galvanic cell. However, for a battery to be of practical use it should be reasonably light, compact and its voltage should not vary appreciably during its use. There are mainly two types of batteries — primary batteries and secondary batteries.
In the primary batteries, the reaction occurs only once and after use over a period of time the battery becomes dead and cannot be reused again, whereas the secondary batteries are rechargeable.
Production of electricity by thermal plants is not a very efficient method and is a major source of pollution. To solve this problem, galvanic cells are designed in such a way that energy of combustion of fuels is directly converted into electrical energy, and these are known as fuel cells. One such fuel cell was used in the Apollo space programme.
Answer the following questions :
(a) How do primary batteries differ from secondary batteries ?
Ans. Primary batteries are not rechargeable while secondary batteries are rechargeable.
(Or any other correct difference)
(b) The cell potential of Mercury cell is 1·35 V, and remains constant during its life. Give reason.
Ans. Overall reaction does not involve any ion in solution whose concentration can change during its lifetime.
(c) Write the reactions involved in the recharging of the lead storage battery.
Ans. Cathode: PbSO4(s) + 2e- → Pb(s) + SO42-(aq)
Anode: PbSO4 (s) + 2H2O (1) → PbO2(s) + SO42-(aq) + 4H+(aq) + 2e-
OR
(c) Write two advantages of fuel cells over other galvanic cells.
Ans. (i) More efficiency, (ii) Pollution free
30. The Valence Bond Theory (VBT) explains the formation, magnetic behaviour and geometrical shapes of coordination compounds whereas "The Crystal Field Theory' for coordination compounds is based on the effect of different crystal fields (provided by ligands taken as point charges), on the degeneracy of d-orbital energies of the central metal atom/ion. The splitting of the d-orbitals provides different electronic arrangements in strong and weak crystal fields. The crystal field theory attributes the colour of the coordination compounds to d-d transition of the electron. Coordination compounds find extensive applications in metallurgical processes, analytical and medicinal chemistry.
Answer the following questions :
(a) What is crystal field splitting energy ?
Ans. The energy used in the splitting of degenerate d-orbitals due to the presence of ligands in a definite geometry is called Crystal Field Splitting Energy.
(b) Give reason for the violet colour of the complex [Ti(H2O)6]3+ on the basis of crystal field theory.
Ans. 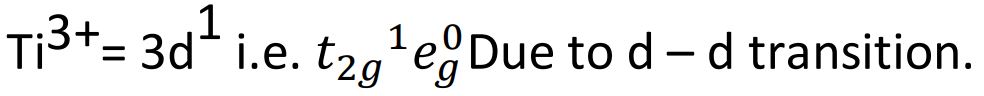
(c) [Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2- is diamagnetic. Explain why.
[Atomic No. : Cr = 24, Ni = 28]
Ans.
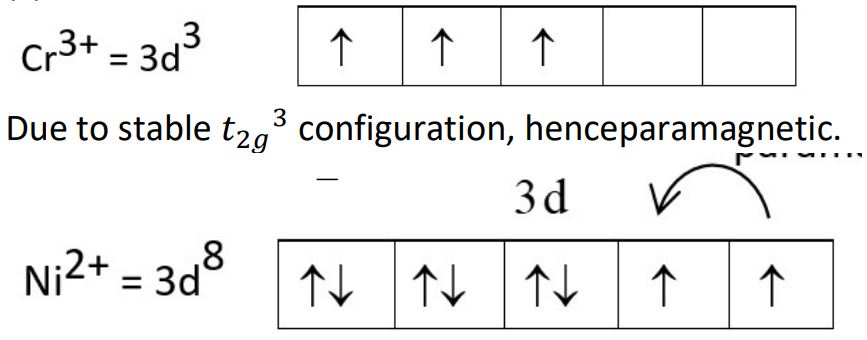
CN- being strong field ligand pair up the electrons and hence diamagnetic.
OR
(c) Explain why [Fe(CN)6]3- is an inner orbital complex, whereas [Fe(H2O)6]3+ is an outer orbital complex.
[Atomic No. : Fe = 26]
Ans. CN- being a strong ligand leads to the pairing of electrons in [Fe(CN)6]³- leading to d²sp³ hybridization.H₂O being a weak ligand does not lead to the pairing of electrons in [Fe(H2O)6]3+ leading to sp³d² hybridization. / In [Fe(CN)6]3-, (n-1)d orbitals of central metal ion are used in hybridization (d²sp³). Hence inner orbital complex whereas in [Fe(H2O)6]3+ n d orbitals of central metal ion are used in hybridization (sp³d²).
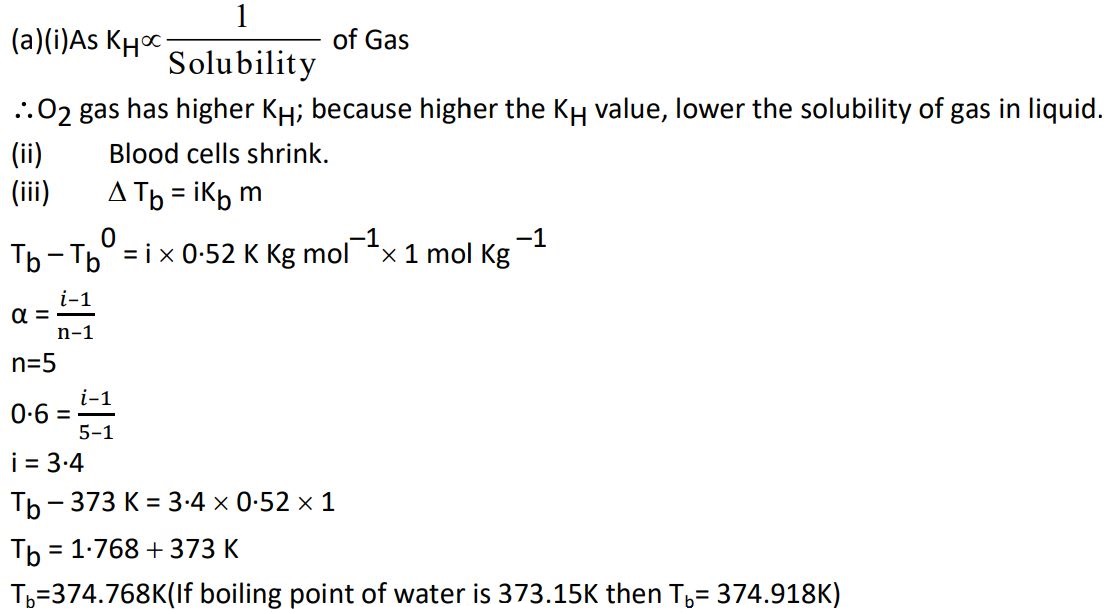
31. (a) (i) At the same temperature, CO2 gas is more soluble in water than O2 gas. Which one of them will have higher value of KH and why ?
(ii) How does the size of blood cells change when placed in an aqueous solution containing more than 0·9% (mass/volume) sodium chloride ?
(iii) 1 molal aqueous solution of an electrolyte A2B3 is 60% ionized. Calculate the boiling point of the solution. (Given : Kb for H2O = 0·52 K kg mol-1)
Ans.
OR
(b) (i) The vapour pressures of A and B at 25ºC are 75 mm Hg and 25 mm Hg, respectively. If A and B are mixed such that the mole fraction of A in the mixture is 0·4, then calculate the mole fraction of B in vapour phase.
Ans.
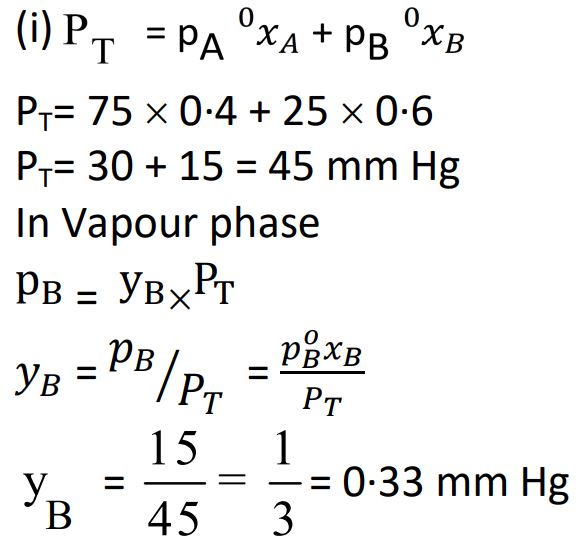
(ii) Define colligative property. Which colligative property is preferred for the molar mass determination of macromolecules ?
Ans. The property which depends upon the number of solute particles but not on the natureof solute.; Osmotic pressure.
(iii) Why are equimolar solutions of sodium chloride and glucose not isotonic ?
Ans. Because sodium chloride undergoes dissociation (i=2) in water while glucose does not./ л= ¡CRT; For NaCl, i=2 and for glucose i=1.
32. Answer any five questions of the following :
(a) N,N-diethyl-benzenesulphonamide is insoluble in alkali. Give reason.
Ans. Because N, N — diethyl-benzenesulphonamide does not contain any hydrogen atom attached to nitrogen atom, it is not acidic, hence insoluble in alkali.
(b) Aniline does not undergo Friedel-Crafts reaction. Why ?
Ans. Due to salt formation with aluminum chloride, the Lewis acid which is used as a catalyst.
(c) Write a simple chemical test to distinguish between methylamine and aniline.
Ans. On reacting with nitrous acid at low temperature aniline forms benzene diazonium chloride which on reacting with phenol forms orange dye whereas methylamine does not. (Or any other suitable chemical test)
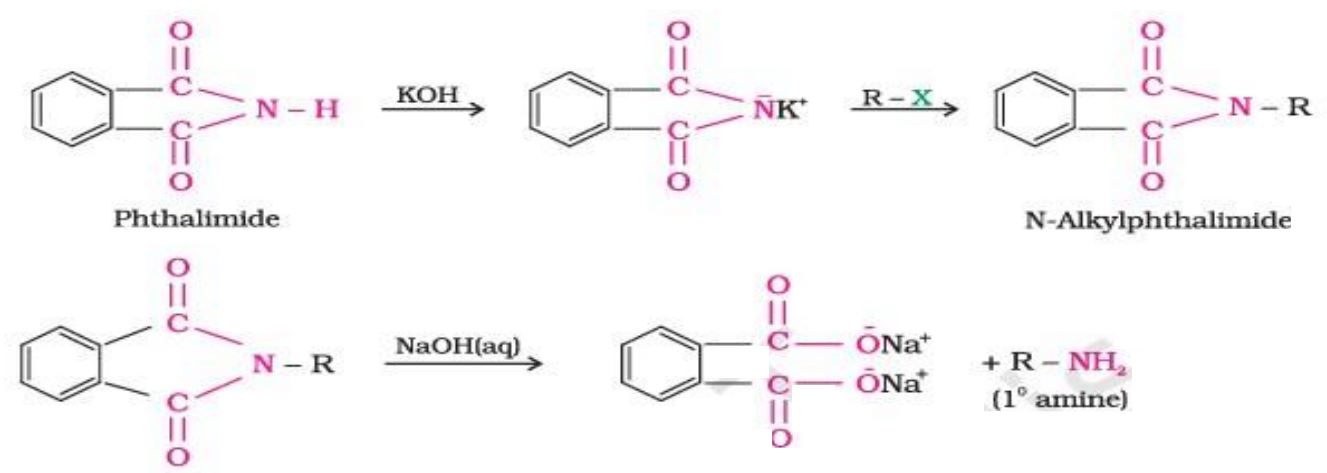
(d) Write the chemical reaction involved in Gabriel phthalimide synthesis.
Ans.
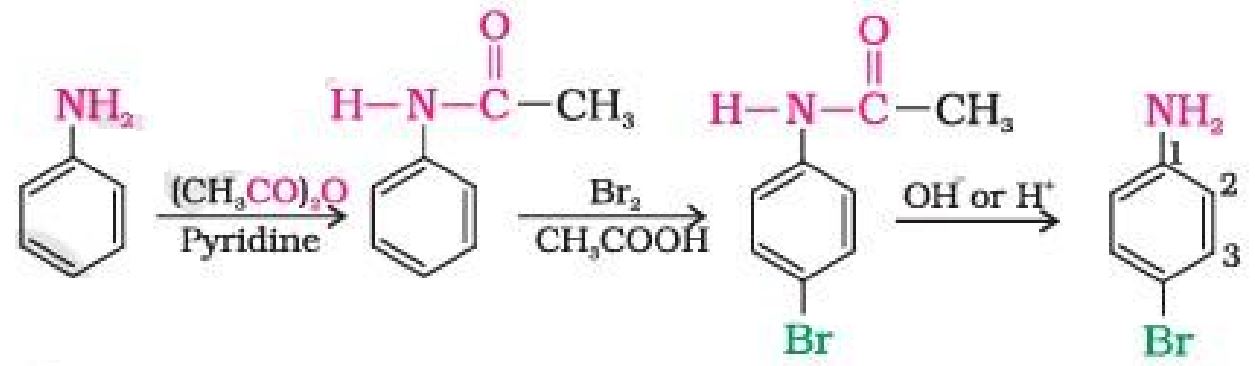
(e) How will you convert aniline to p-bromoaniline ?
Ans.
(f) Complete the following reaction :
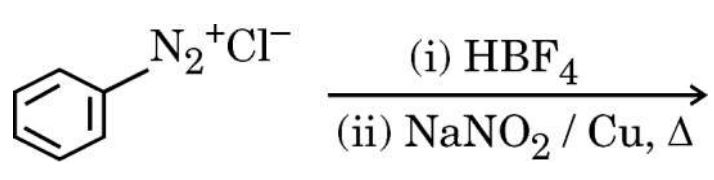
Ans.
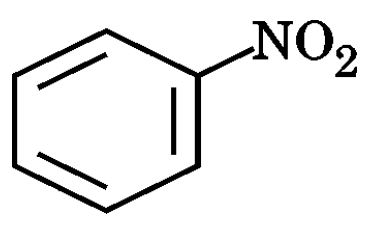
(g) Write the structures of A and B in the following reaction :
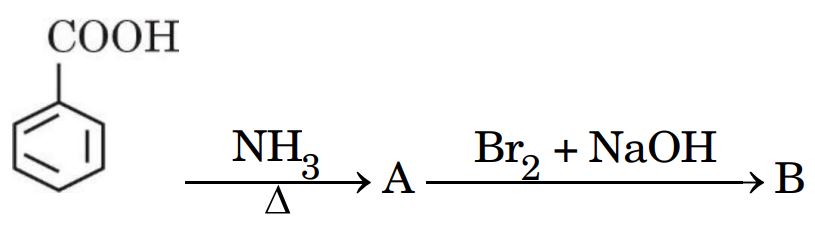
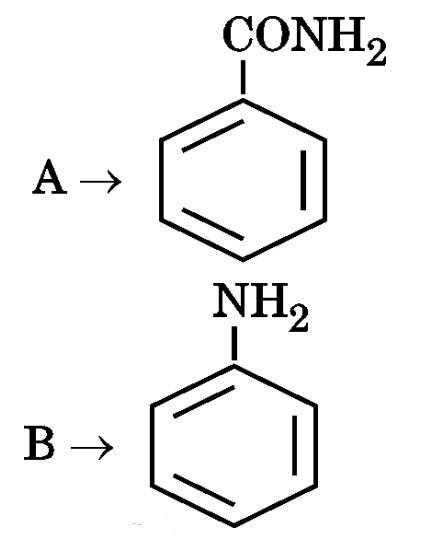
Ans.
(ANY FIVE)
33. (a) (i) Account for the following :
(1) The melting and boiling points of Zn, Cd and Hg are low.
Ans. Because of the absence of unpaired electrons in their d-orbitals resulting in weak bonding between the atoms/ due to presence of fully filled d-orbitals, weak metallic bonding takes place.
(2) Of the d4 species, Cr2+ is strongly reducing while Mn3+ is strongly oxidizing.
Ans.

(3) Eo value of Cu2+/Cu is + 0·34 V.
Ans.
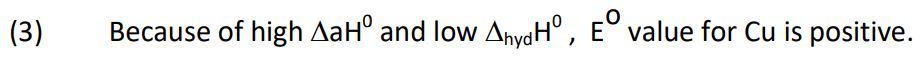
(ii) Complete and balance the following chemical equations :
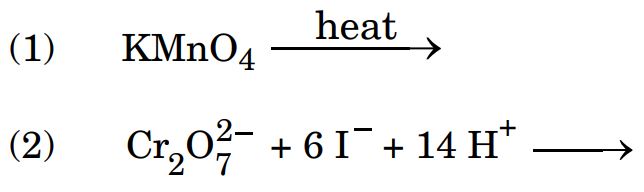
Ans.
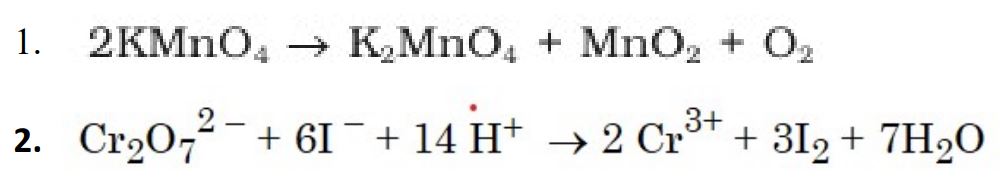
(b) (i) Out of Cu2Cl2 and CuCl2, which is more stable in aqueous solution and why ?
(ii) Write the general electronic configuration of f-block elements.
(iii) Predict which of the following will be coloured in aqueous solution and why ?
Sc3+, Fe3+, Zn2+
[Atomic number : Sc = 21, Fe = 26, Zn = 30]
(iv) How can you obtain potassium dichromate from sodium chromate ?
(v) Why do transition metals and their compounds show catalytic activities ?
Ans.
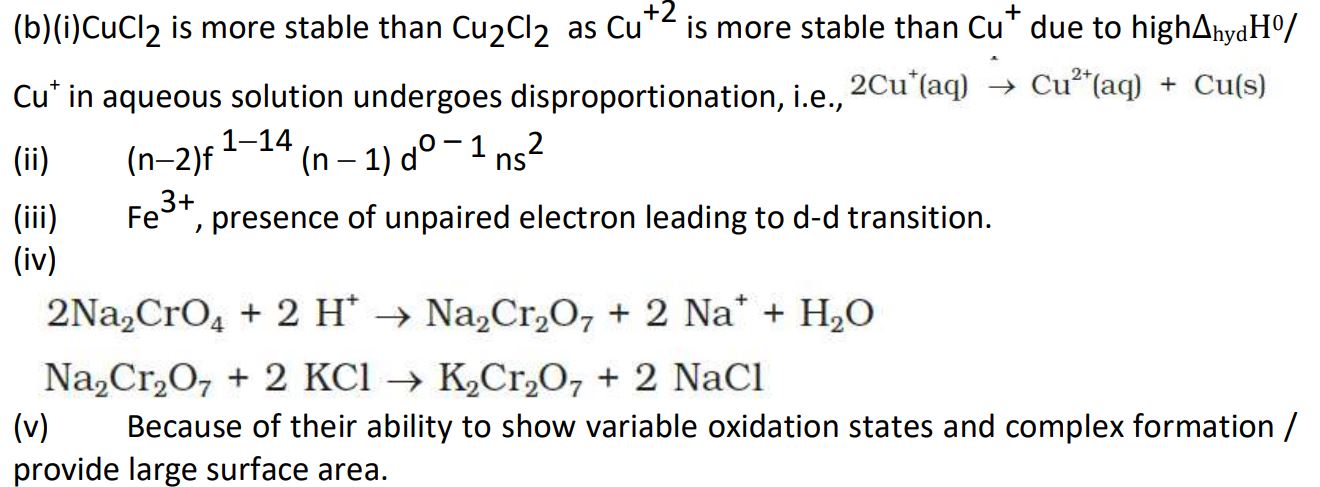
-
👉 Read Also - CBSE Class 12 Half-Yearly/Mid Term 2024-25 : Most Important Questions with Answers; PDF Download (All Subjects)
👉 Read Also - How CBSE’s New Exam Pattern Will Impact Class 11 and 12 Students
👉 CBSE Class 12 Study Materials
| CBSE Class 12 Syllabus 2024-25 | CBSE Class 12 Previous Year Papers |
| NCERT Books For Class 12 Books | NCERT Class 12 Solutions |
| CBSE Class 12 Full Study Material | CBSE Class 12 Sample Paper 2024-25 |







 Profile
Profile Signout
Signout








 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










