Dioxoanion is better hydride donor. Electron donating group at ortho/para position further promote H- transfer.
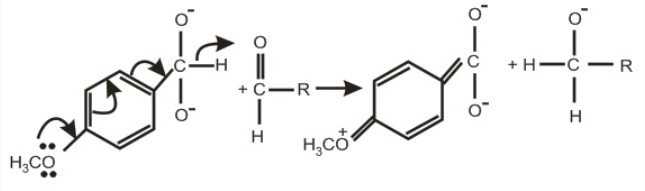
Concepts :
Main Concept :
Preparation of Carboxylic Acids from Cannizzaro's ReactionCannizzaro reaction
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction that involves the base-induced disproportionation of an aldehyde lacking a hydrogen atom in the alpha position.
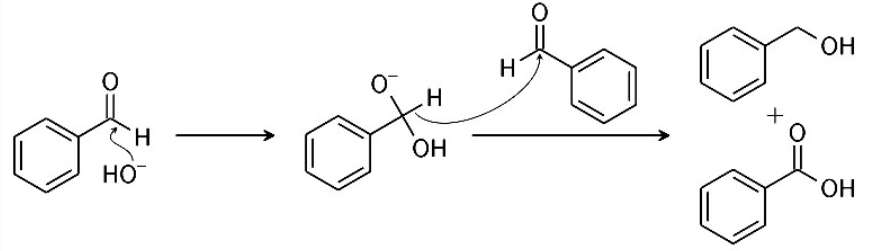
![]()
The oxidation product is a salt of a carboxylic acid and the reduction product is an alcohol.
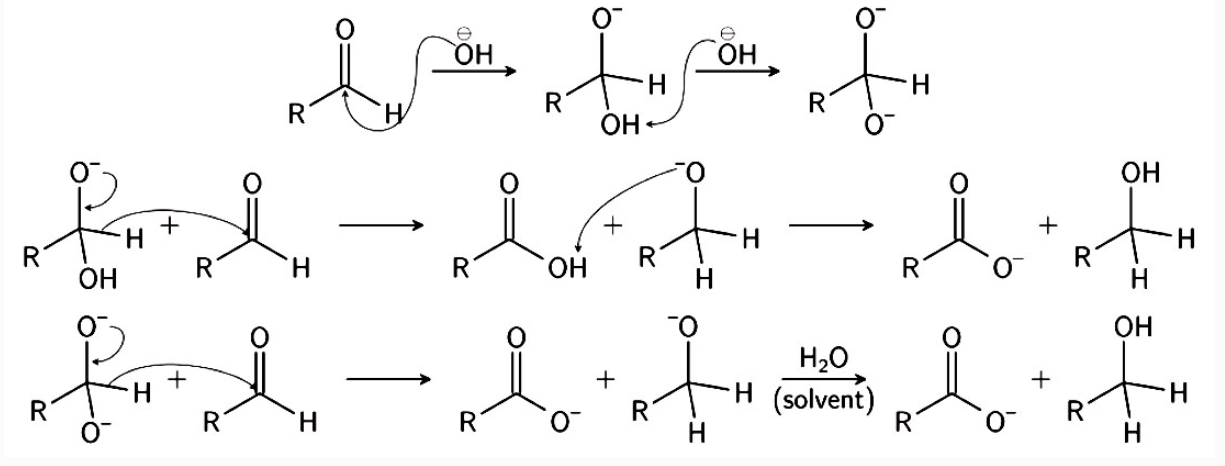
Mechanism
The reaction involves a nucleophilic acyl substitution on an aldehyde, with the leaving group concurrently attacking another aldehyde in the second step. First, hydroxide attacks a carbonyl. The resulting tetrahedral intermediate then collapses, re-forming the carbonyl and transferring hydride to attack another carbonyl. In the final step of the reaction, the acid and alkoxide ions formed exchange a proton. In the presence of a very high concentration of base, the aldehyde first forms a doubly charged anion from which a hydride ion is transferred to the second molecule of aldehyde to form carboxylate and alkoxide ions. Subsequently, the alkoxide ion acquires a proton from the solvent.
Overall, the reaction follows third-order kinetics. It is second order in aldehyde and first order in base:
Rate \(=\mathrm{k}[\mathrm{RCHO}]^{2}\left[\mathrm{OH}^{-}\right]\)
At very high base a second path (k') becomes important that is second order in base:
Rate=k[RCHO]2[OH−]+k'[RCHO]2[OH−]2
The k' pathway implicates a reaction between the doubly charged anion \(\left(\mathrm{RCHO}_{2}^{2-}\right)\)and the aldehyde. The direct transfer of hydride ion is evident from the observation that the recovered alcohol does not contain any deuterium attached to the ![]() -carbon when the reaction is performed in the presence of D2O.
-carbon when the reaction is performed in the presence of D2O.
Hence, the correct option is (D)


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















