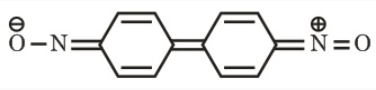
Complete octet & Extended conjugation
Concepts :
Main Concept :
Stability of resonance structuresExample
The above resonance structures show that the electrons are delocalized within the molecule and through this process the molecule gains extra stability. Ozone with both of its opposite charges creates a neutral molecule and through resonance it is a stable molecule. The extra electron that created the negative charge on either terminal oxygen can be delocalized by resonance through the terminal oxygens.
Benzene is an extremely stable molecule and it is accounted for its geometry and molecular orbital interaction, but most importantly it's due to its resonance structures. The delocalized electrons in the benzene ring make the molecule very stable and with its characteristics of a nucleophile, it will react with a strong electrophile only and after the first reactivity, the substituted benzene will depend on its resonance to direct the next position for the reaction to add a second substituent.
The next molecule, the Amide, is a very stable molecule that is present in most biological systems, mainly in proteins. By studies of NMR spectroscopy and X-Ray crystallography it is confirmed that the stability of the amide is due to resonance which through molecular orbital interaction creates almost a double bond between the Nitrogen and the carbon.
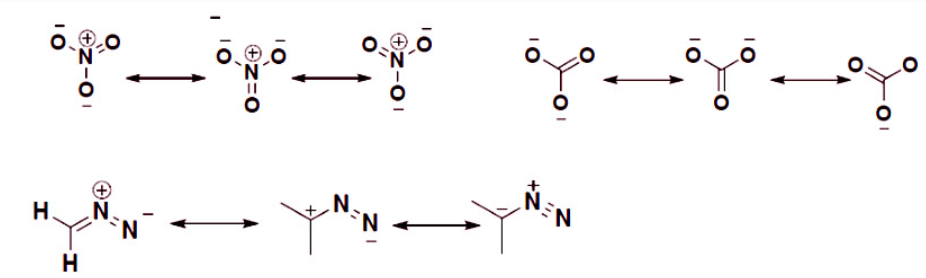
Example: Multiple Resonance of other Molecules
![]()
Molecules with more than one resonance form
Some structural resonance conformations are the major contributor or the dominant forms that the molecule exists. For example, if we look at the above rules for estimating the stability of a molecule, we see that for the third molecule the first and second forms are the major contributors for the overall stability of the molecule. The nitrogen is more electronegative than carbon so, it can handle the negative charge more than carbon. A carbon with a negative charge is the least favorable conformation for the molecule to exist, so the last resonance form contributes very little for the stability of the Ion.
Hybrid resonance
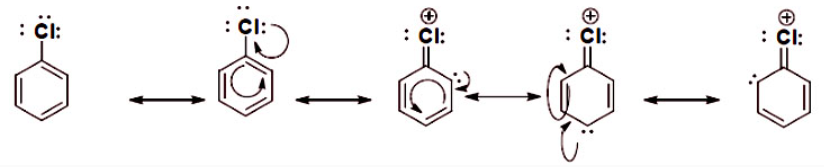
The different resonance forms that the molecule has helps and directs the reactivity to specific sites.
The Hybrid Resonance forms show the different Lewis structures with the electron been delocalized. This is very important for the reactivity of chloro-benzene because in the presence of an electrophile it will react and the formation of another bond will be directed and determine by resonance. The long pair of electrons delocalized in the aromatic substituted ring is where it can potentially form a new bond with an electrophile, as it is shown there are three possible places that reactivity can take place, the first to react will take place at the para position with respect to the chloro substituent and then to either ortho position.
Hence, the correct option is (D)


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















