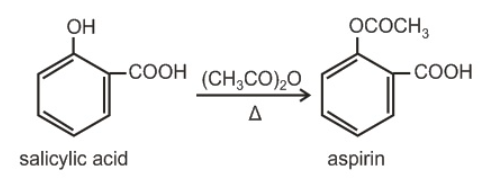
Acetyl salicylic acid is known as aspirin.
![]()
Concepts :
Main Concept :
Examples on Preparation of esters by Fischer esterification![]()
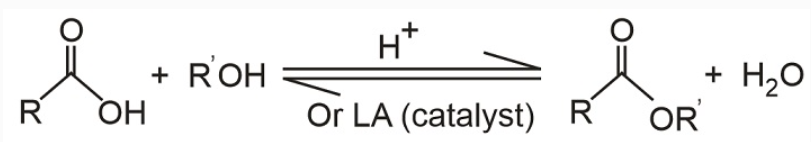
The Lewis or Brønsted acid-catalyzed esterification of carboxylic acids with alcohols to give esters is a typical reaction in which the products and reactants are in equilibrium.
The equilibrium may be influenced by either removing one product from the reaction mixture (for example, removal of the water by azeotropic distillation or absorption by molecular sieves) or by employing an excess of one reactant.
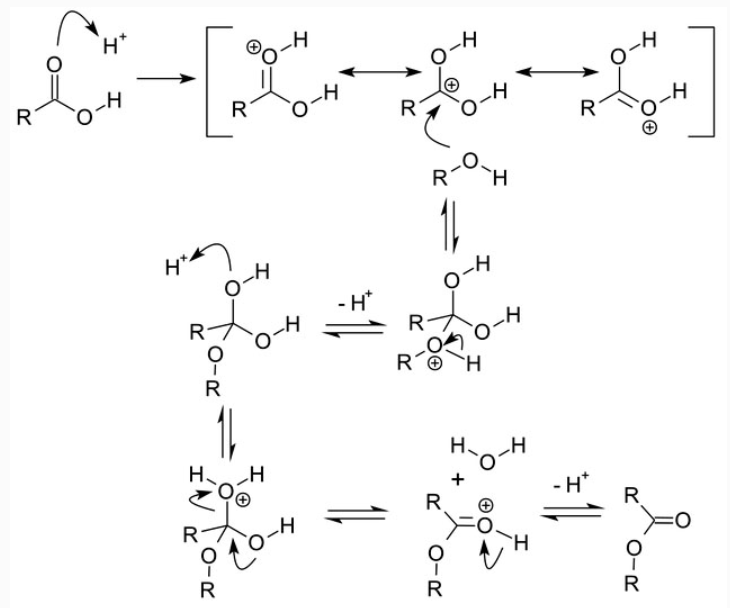
Mechanism of the Fischer Esterification
Addition of a proton or a Lewis acid leads to a more reactive electrophile. Nucleophilic attack of the alcohol gives a tetrahedral intermediate in which there are two equivalent hydroxyl groups. One of these hydroxyl groups is eliminated after a proton shift (tautomerism) to give water and the ester.
Alternative reactions employ coupling reagents such as DCC (Steglich Esterification), preformed esters (transesterification), carboxylic acid chlorides or anhydrides (see overview). These reactions avoid the production of water. Another pathway for the production of esters is the formation of a carboxylate anion, which then reacts as a nucleophile with an electrophile (similar reactions can be found here). Esters may also be produced by oxidations, namely by the Baeyer-Villiger oxidation andoxidative esterifications.
Alternative reactions employ coupling reagents such as DCC (Steglich Esterification), preformed esters (transesterification), carboxylic acid chlorides or anhydrides (see overview). These reactions avoid the production of water. Another pathway for the production of esters is the formation of a carboxylate anion, which then reacts as a nucleophile with an electrophile (similar reactions can be found here). Esters may also be produced by oxidations, namely by the Baeyer-Villiger oxidation and oxidative esterifications.
Hence, the correct option is (A)


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















