The second ionization potential values of Cu and Cr are sufficiently higher than those of neighbouring elements . This is because of the electronic configuration of Cu+ which is 3d10 (completely filled) and of Cr+ which is 3 d5 (half filled ),i.e for the second ionization potentials , the electron is to be removed from very stable configuration.
Concepts :
Main Concept :
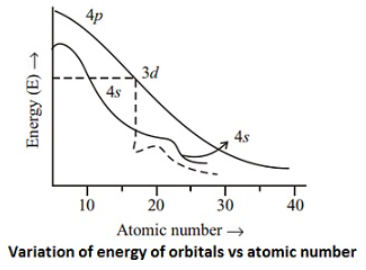
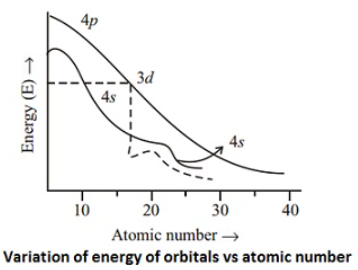
Electronic configurations of d-block elementsThe general electronic configuration of transition elements is (n-1) d1-10 ns1-2. The (n-1) stands for inner shell and the d-orbitals may have one to ten electrons and the s-orbital of the outermost shell (n) may have one or two electrons. It is observed from the Figure that 4s orbital (l = 0 and n = 4) is of lower energy than 3d orbitals (l = 2 and n = 3) upto potassium (At. No.19). The energy of both these orbitals is almost same in case of calcium (At. No. 20), but the energy of 3d orbitals decreases with further increase of nuclear charge and becomes lower than 4s, and 4p, (in case of scandium At. No.21). Thus after filling of 4s orbital successively with two electrons at atomic number 19 and 20, the next incoming electron goes to 3d orbital instead of 4p, as the former is of lower energy than the latter. This means that 21st electron enters the underlying principal quantum level with n = 3 rather than the outermost level with n = 4 which started filling at potassium (At. No.19), the first element of the fourth period. In the case of next nine elements following calcium, the incoming electron is filled in the d-subshell. Since half filled and completely filled subshells are stabler than the one in which one electron is short, an electron gets transferred from 4s to 3d in case of the elements with atomic number 24 and 29. Consequently, configuration of chromium and copper have only one 4s electron.
Electronic configuration of first series( or 3d) transition elements
| Element |
Symbol |
Z |
Electronic Configuration |
| Scandium |
Sc |
21 |
1s2 2s2 2p6 3s2 3p6 3d1 4s2 |
| Titanium |
Ti |
22 |
1s2 2s2 2p6 3s2 3p6 3d2 4s2 |
| Vanadium |
V |
23 |
1s2 2s2 2p6 3s2 3p6 3d3 4s2 |
| Chromium |
Cr |
24 |
1s2 2s2 2p6 3s2 3p6 3d5 4s1 |
| Manganese |
Mn |
25 |
1s2 2s2 2p6 3s2 3p6 3d5 4s2 |
| Iron |
Fe |
26 |
1s2 2s2 2p6 3s2 3p6 3d6 4s2 |
| Cobalt |
Co |
27 |
1s2 2s2 2p6 3s2 3p6 3d7 4s2 |
| Nickel |
Ni |
28 |
1s2 2s2 2p6 3s2 3p6 3d8 4s2 |
| Copper |
Cu |
29 |
1s2 2s2 2p6 3s2 3p6 3d10 4s1 |
| Zinc |
Zn |
30 |
1s2 2s2 2p6 3s2 3p6 3d10 4s2 |
The general electronic configuration of transition elements is (n-1) d1-10 ns1-2. The (n-1) stands for inner shell and the d-orbitals may have one to ten electrons and the s-orbital of the outermost shell (n) may have one or two electrons. It is observed from the Figure that 4s orbital (l = 0 and n = 4) is of lower energy than 3d orbitals (l = 2 and n = 3) upto potassium (At. No.19). The energy of both these orbitals is almost same in case of calcium (At. No. 20), but the energy of 3d orbitals decreases with further increase of nuclear charge and becomes lower than 4s, and 4p, (in case of scandium At. No.21). Thus after filling of 4s orbital successively with two electrons at atomic number 19 and 20, the next incoming electron goes to 3d orbital instead of 4p, as the former is of lower energy than the latter. This means that 21st electron enters the underlying principal quantum level with n = 3 rather than the outermost level with n = 4 which started filling at potassium (At. No.19), the first element of the fourth period. In the case of next nine elements following calcium, the incoming electron is filled in the d-subshell. Since half filled and completely filled subshells are stabler than the one in which one electron is short, an electron gets transferred from 4s to 3d in case of the elements with atomic number 24 and 29. Consequently, configuration of chromium and copper have only one 4s electron.
Electronic configuration of first series( or 3d) transition elements
| Element |
Symbol |
Z |
Electronic Configuration |
| Scandium |
Sc |
21 |
1s2 2s2 2p6 3s2 3p6 3d1 4s2 |
| Titanium |
Ti |
22 |
1s2 2s2 2p6 3s2 3p6 3d2 4s2 |
| Vanadium |
V |
23 |
1s2 2s2 2p6 3s2 3p6 3d3 4s2 |
| Chromium |
Cr |
24 |
1s2 2s2 2p6 3s2 3p6 3d5 4s1 |
| Manganese |
Mn |
25 |
1s2 2s2 2p6 3s2 3p6 3d5 4s2 |
| Iron |
Fe |
26 |
1s2 2s2 2p6 3s2 3p6 3d6 4s2 |
| Cobalt |
Co |
27 |
1s2 2s2 2p6 3s2 3p6 3d7 4s2 |
| Nickel |
Ni |
28 |
1s2 2s2 2p6 3s2 3p6 3d8 4s2 |
| Copper |
Cu |
29 |
1s2 2s2 2p6 3s2 3p6 3d10 4s1 |
| Zinc |
Zn |
30 |
1s2 2s2 2p6 3s2 3p6 3d10 4s2 |
Ionisation energies of transition elementsIonisation energies : The ionisation energies of the elements of first transition series are are given below:
| Elements |
l1 |
l2 |
l3 |
| Sc |
632 |
1245 |
2450 |
| Ti |
659 |
1320 |
2721 |
| V |
650 |
1376 |
2873 |
| Cr |
652 |
1635 |
2994 |
| Mn |
716 |
1513 |
3258 |
| Fe |
762 |
1563 |
2963 |
| Co |
758 |
1647 |
3237 |
| Ni |
736 |
1756 |
3400 |
| Cu |
744 |
1961 |
3560 |
| Zn |
906 |
1736 |
3838 |
* in kJ mol-1
The following generalizations can be obtained from the ionisation energy values given above.
(i) The ionisation energies or these elements are high and in the most cases lie those of s- and p-block elernents. This indicates that the transition elements are less electropositive than s-block elements.
Explanation : Transition metals have smaller atomic radii and higher nuclear charge as compared to the alkali metals. Both these factors tend to increase the ionisation energy, as observed.
(ii) The ionisation energy in any transition series increases in the nuclear with atomic number; the increase however is not smooth and as sharp as seen in the case of s and p-block elernents.
Explanation : The ionisation energy increases due to the increase in the nuclear charge with atomic number at the beginning of the series. Gradually, the shielding effect of the added electrons also increases. This shielding effect tends to decrease the attraction due to the nuclear charge. These two opposing factors lead to a rather gradual increase in the ionisation energies in any transition series.
(iii)The first ionisation energies of 5d-series of elements are much higher than those of the 3d and 4d series elements.
Explanation : In the 5d-series of transitions elements, after lanthanum (La), the added electrons to the next inner 4f orbitals. The 4f electrons have shielding effect. As a result, the outermost electrons experience greater nuclear attraction. This leads to higher ionisation energies for the 5d- series of transition elements.Ionisation energies : The ionisation energies of the elements of first transition series are are given below:
| Elements |
l1 |
l2 |
l3 |
| Sc |
632 |
1245 |
2450 |
| Ti |
659 |
1320 |
2721 |
| V |
650 |
1376 |
2873 |
| Cr |
652 |
1635 |
2994 |
| Mn |
716 |
1513 |
3258 |
| Fe |
762 |
1563 |
2963 |
| Co |
758 |
1647 |
3237 |
| Ni |
736 |
1756 |
3400 |
| Cu |
744 |
1961 |
3560 |
| Zn |
906 |
1736 |
3838 |
* in kJ mol-1
The following generalizations can be obtained from the ionisation energy values given above.
(i) The ionisation energies or these elements are high and in the most cases lie those of s- and p-block elernents. This indicates that the transition elements are less electropositive than s-block elements.
Explanation : Transition metals have smaller atomic radii and higher nuclear charge as compared to the alkali metals. Both these factors tend to increase the ionisation energy, as observed.
(ii) The ionisation energy in any transition series increases in the nuclear with atomic number; the increase however is not smooth and as sharp as seen in the case of s and p-block elernents.
Explanation : The ionisation energy increases due to the increase in the nuclear charge with atomic number at the beginning of the series. Gradually, the shielding effect of the added electrons also increases. This shielding effect tends to decrease the attraction due to the nuclear charge. These two opposing factors lead to a rather gradual increase in the ionisation energies in any transition series.
(iii)The first ionisation energies of 5d-series of elements are much higher than those of the 3d and 4d series elements.
Explanation : In the 5d-series of transitions elements, after lanthanum (La), the added electrons to the next inner 4f orbitals. The 4f electrons have shielding effect. As a result, the outermost electrons experience greater nuclear attraction. This leads to higher ionisation energies for the 5d- series of transition elements.
Hence, the correct option is (D)


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















