The energy of a photon corresponding to \(\lambda=100 \mathrm{nm}\) is
\frac{1242 \mathrm{eV} \mathrm{nm}}{100 \mathrm{nm}}=12 \cdot 42 \mathrm{eV}
and the corresponding to \(\lambda=200 \mathrm{nm}\) is \(6.21 \mathrm{eV}\). The energy needed to take the atom from the ground state to the first excited state is
\mathrm{E}_{2}-\mathrm{E}_{1}=13.6 \mathrm{eV}-3.4 \mathrm{eV}=10.2 \mathrm{eV}
to the second excited state is
\mathrm{E}_{3}-\mathrm{E}_{1}=13.6 \mathrm{eV}-1.5 \mathrm{eV}=12.1 \mathrm{eV}
to the third excited state is
\mathrm{E}_{4}-\mathrm{E}_{1}=13.6 \mathrm{eV}-0.85 \mathrm{eV}=12.75 \mathrm{eV}, \text { etc. }
Thus, \(10.2 \mathrm{eV}\) photons and \(12.1 \mathrm{eV}\) photons have large probability of being absorbed from the given range \(6.21 \mathrm{eV}\) to \(12.42 \mathrm{eV} .\) The corresponding wavelengths are
and
\begin{array}{c}
\lambda_{1}=\frac{1242 \mathrm{eV} \mathrm{nm}}{10 \cdot 2 \mathrm{eV}}=122 \mathrm{nm} \\
\lambda_{2}=\frac{1242 \mathrm{eV}}{12 \cdot 1 \mathrm{eV}}=103 \mathrm{nm}
\end{array}
These wavelengths will have low intensity in the transmitted beam.
Concepts:
Main Concept:
Bohr's PostulatesBohr Model:
Niels Bohr applied classical mechanics, electromagnetism and Planck's quantum theory to modify the Rutherford's model and proposed his atomic model in 1913 . His model gives satisfactory explanation of stable atomic structure and emission of line spectra by hydrogen atom. He presented his theory in the form of 3 postulates:
Postulate 1: The electron in a hydrogen atom revolves in circular orbit around the nucleus with nucleus at the centre of orbit. The necessary centripetal force for circular motion is provided by electrostatic force of attraction between the positively charged nucleus and negatively charged electron.
Hydrogen atom consists of only one electron and the nucleus. Let an electron of mass \(\mathrm{m}\) revolves with speed \(\mathrm{v}\) in an orbit of radius r. the charge on nucleus must be te to make atom electrically neutral.
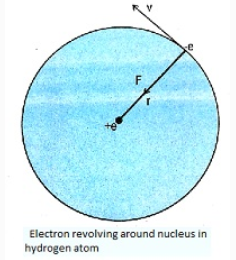
The force of attraction between electron and nucleus is given by Coulomb's law:
Postulate 2 :
The electron revolves around the nucleus only in those orbits for which the angular momentum is equal to an integral multiple of \(\mathrm{h} / 2 \pi,\) where \(\mathrm{h}\) is Planck's constant. These orbits are called stable or stationary (or permitted or quantized or Bohr orbits) and electron does not radiate energy whole revolving in these orbits i.e. its energy remains constant and hence stability of atomic structure. The force of attraction between electron and nucleus is given by Coulomb's law:
\(\mathrm{F}=\frac{1}{4 \pi \varepsilon_{0}} \frac{\rho^{2}}{\mathrm{r}^{2}}\)
The centripetal force is given by \(\frac{\mathrm{mv}^{2}}{\mathrm{r}}\)
\(\therefore\) Centripetal force \(=\) Electrostatic force of attraction.
\(\frac{\mathrm{mv}^{2}}{\mathrm{r}}=\frac{1}{\pi \varepsilon_{0}} \frac{\mathrm{e}^{2}}{\mathrm{r}^{2}}\)
That is electrons revolve in only those orbits that satisfies the equation. The integer 'n' is called principal quantum number. It also designates the orbit number.
Hence, the correct option is (B)


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















