CBSE 12th exams are underway and your CBSE 12th Chemistry exam is scheduled on 27th Feb, 2025. You have just a few hours left for CBSE 12th Chemistry exam.
We know how important it is to focus on the right topics and questions during revision, so this article provides important CBSE 12th Chemistry Very Short Questions along with Answers for last minute revision.
👉 Read Also - CBSE 12 Chemistry Exam 2025 : Top 50 MCQs with Answers for Last-Minute Revision - Download PDF
Get most repeated questions for CBSE Class 12 Chemistry. These questions are important and often appear in exams. Practice them well to prepare for the CBSE Class 12 Chemistry Exam 2025.
Most Very Short Type Questions in CBSE Class 12 Chemistry Exam 2025
1. Define Molarity.
Answer ⇒ Molarity is the number of moles of solute dissolved in one litre of solution.
2. Define Mole fraction.
Answer ⇒ Mole fraction is the ratio of number of moles of one component to the total number of moles in a mixture.
3. Define Osmotic pressure.
Answer ⇒ The minimum excess pressure that has to be applied on the solution to prevent the entry of the solvent into the solution through a semi-permeable membrane is called osmotic pressure.
4. What type of intermolecular attractive interaction exists in the pair of methanol and acetone? (2014 Delhi)
Answer ⇒ Solute-solvent dipolar interactions exist in the pair of methanol and acetone.
👉 Read Also - CBSE 12th Chemistry Exam 2025 : Most Important Assertion Reason Questions with Answers for Last Minute Revision
5. What is meant by ‘reverse osmosis’? (2010 All India)
Answer ⇒ If a pressure higher than the osmotic pressure is applied on the solution, the solvent will flow from the solution into the pure solvent through a semipermeable membrane. This process is called reverse osmosis (RO).
6. What are isotonic solutions? (2014 Delhi)
Answer ⇒ An isotonic solution is a kind of solution with the same salt concentration as blood and cells. Those solutions which exert the same osmotic pressure under similar conditions (For example, 0.9% NaCl solution by mass volume is isotonic with human blood).
7. What is meant by Colligative properties?
Answer ⇒ Those properties of ideal solutions which depend only on the number of particles of the solute dissolved in a definite amount of the solvent and do not depend on the nature of solute are called colligative properties.
8. Conductivity of CH3COOH decreases on dilution. Explain. (CBSE 2018)
Answer ⇒ Conductivity of CH3COOH decreases on dilution because the number of H⁺ ions decreases per unit volume of a solution.
9. Mercury cell delivers a constant potential during its lifetime. Give reason. 2023 (Series: HFG1E/2)
Answer ⇒ Mercury cell delivers a constant potential during its lifetime because the overall reaction does not involve any ion in solution whose concentration can change.
10. Define ‘rate of a reaction’. (2010 Delhi, 2010 AI, 2016 Comptt. Delhi, 2009 Delhi)
Answer ⇒ Rate of a reaction: The change in the concentration of any one of the reactants or products per unit time is called the rate of a reaction.
In other words, the rate of a chemical reaction is the change in the molar concentration of the species taking part in a reaction per unit time.
👉 Read Also - CBSE Class 12 Chemistry 2025: Chapter-Wise Competency-Based Questions with Solutions – Free PDF Download
11. Define rate constant (K). (2016 Comptt. All India, 2014 D, 2014 Comptt. D, 2015 Comptt. D)
Answer ⇒ Rate constant: It is defined as the rate of reaction when the concentration of reactants is taken as unity.
12. What is meant by 'lanthanoid contraction'?
Answer ⇒ The steady decrease in the ionic radius from La3+ to Lu3+ is termed as lanthanoid contraction.
13. Why do transition elements show variable oxidation states? (2014, 2013, 2015, 2016 Comptt. D, 2014 CAI, 2016 D, 2013 AI)
Answer ⇒ The variability of oxidation state of transition elements is due to incompletely filled d-orbitals and presence of unpaired electrons, i.e., (n)s and (n - 1)d electrons have approximate equal energies.
14. Explain the following observations:
Transition elements generally form coloured compounds. (2010 Delhi, 2011 D, 2012 D, 2014 AI, 2015 D, 2017 CAI, 2019 BVM/1)
Answer ⇒ Because of the presence of unpaired d electrons, which undergo d-d transition by absorption of energy, and then the emitted light falls in the visible region showing complementary colours. This is how transition elements form coloured compounds.
15. Assign reason for the following:
Both O2 and F2 stabilize high oxidation states of transition metals, but the ability of oxygen to do so exceeds that of fluorine. (2014 Comptt. All India)
Answer ⇒ The ability of O2 to stabilize higher oxidation states exceeds that of fluorine because oxygen can form multiple bonds with metals.
👉 Read Also - CBSE Class 12 Chemistry Exam 2025: Important Questions, PYQs & Sample Papers for All Chapters - Free PDF Download
16. Mn shows the highest oxidation state of+7 with oxygen but with fluorine it showsthe highest oxidation state of +4. Explain. (2016 AI, 2016 D)
Answer ⇒ Because oxygen stabilizes the highestoxidation state (+7 of Mn) even more thanfluorine i.e., +4 since oxygen has the abilityto form multiple bonds with metal atoms.
17. What is meant by chelate effect? (2015 Comptt. All India)
Answer ⇒ Chelate effect: When a bidentate or a polydentate ligand contains donor atoms positioned in such a way that when they coordinate with the central metal ion, a five or a six-membered ring is formed. This effect is called the Chelate effect. As a result, the stability of the complex increases.
Example: The complex of Ni2+ with 'ion' is more stable than NH3.
18. Why are low spin tetrahedral complexes not formed? (2017 Comptt. Delhi, 2017 AI)
Answer ⇒ Low spin tetrahedral complexes are rarely observed because orbital splitting energies for tetrahedral complexes are sufficiently large for forcing pairing.
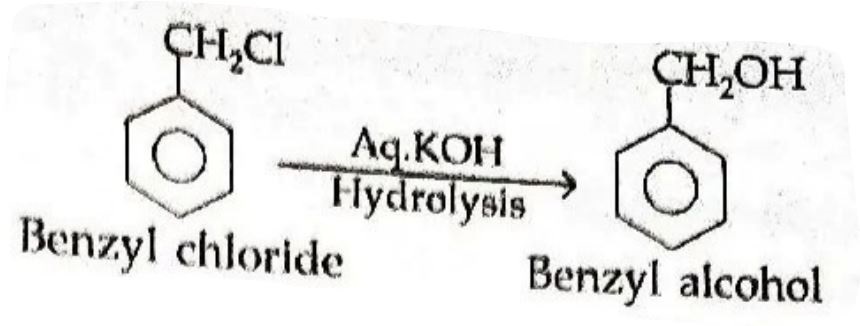
19. How is the following conversion carried out? (2010 Delhi)
Benzyl chloride to benzyl alcohol
Answer ⇒ Benzyl chloride to benzyl alcohol
20. Write one stereochemical difference between SN1 and SN2 reactions. (2019 BVM/1)
Answer ⇒ SN1 reactions lead to the formation of racemic mixture whereas SN2 reactions lead to inversion in configuration of product.
👉 Read Also - CBSE Board Class 12 Chemistry Exam 2025 : Chapter-Wise Most Predicted Questions with Answers; Download Free PDF
21. Why is t-butyl bromide more reactive towards SN1 reaction as compared to n-butyl bromide? (2019 BVM/4)
Answer ⇒ t-butyl bromide is more reactive towards SN1 reaction as compared to n-butyl bromide because it is a tertiary halide and produces higher stability of 3° carbocation.
22. Haloalkanes easily dissolve in organic solvents, why? (2011 Delhi)
Answer ⇒ Because the new forces of attraction set up between haloalkanes and solvent molecules are of the same strength as the forces of attraction being broken.
23. n-Butyl bromide has higher boiling point than t-butyl bromide. Explain. (2015 Delhi)
Answer ⇒ n-Butyl bromide has higher boiling point than t-butyl bromide because it has larger surface area, hence have more Van der Waals' forces.
24. o-Nitrophenol has lower b.p. than p-nitrophenol. Explain. (2013 Comptt. Delhi)
Answer ⇒ Ortho-nitrophenol has lower boiling point due to formation of intramolecular H-bonding, whereas p-nitrophenol forms intermolecular H-bonding.
25. Why p-dichlorobenzene has higher melting point than those of ortho-and meta-isomers? (2023 Series: HFG1E/2, 2019 BVM/4)
Answer ⇒ p-Isomers are comparatively more symmetrical and fit closely in the crystal lattice, thus require more heat to break these strong forces of attraction. Therefore, higher melting point than o- and m-isomers.
👉 Read Also - CBSE Class 12 Chemistry Board Exam 2025 : Most Repeated Questions from Last 10 Years; Download PDF
26. The C—O bond is much shorter in phenol than in ethanol. Give reason. (2012 Comptt. Delhi)
Answer ⇒ Carbon of C—O bond of phenol is sp² hybridised, so it acquires a partial double bond character but in ethanol it is sp³ hybridised and a single bond. Double bond is shorter than a single bond.
27. The C—O—H bond angle in alcohols is slightly less than the tetrahedral angle (109°28'). Explain. (2015 All India)
Answer ⇒ The C—O—H bond angle in alcohols is slightly less than tetrahedral angle due to slightly less than tetrahedral angle due to repulsion between the lone pairs of electrons of oxygen.
28. Why is the C—O bond length in phenols less than that in methanol? (2023 Series: HFG1E/5)
Answer ⇒ The C—O bond length in phenol is slightly less than that in methanol due to partial double bond character of C—O bond because of the conjugation of lone pair of electrons of oxygen with the aromatic ring.
29. Ortho nitrophenol has lower boiling point than p-nitrophenol. Why? (2012 CD)
Answer ⇒ Ortho-nitrophenol has lower boiling point due to formation of intramolecular H-bonding whereas p-nitrophenol forms intermolecular H-bonding.
30. Ortho-nitrophenol is more acidic than ortho-methoxyphenol. Why? (2012 Comptt. Delhi)
Answer ⇒ NO2 group is an electron withdrawing group while methoxy group is electron donating in nature. The release of H⁺ is easier from O-nitrophenol while it is difficult from O methoxyphenol.
31. Ethanal is soluble in water. Why? (2013 All India)
Answer ⇒ Ethanal is soluble in water due to H-bonding between the polar carbonyl group and water molecules.
👉 CBSE Class 12 Study Materials
| CBSE Class 12 Syllabus 2024-25 | CBSE Class 12 Previous Year Papers |
| NCERT Books For Class 12 Books | NCERT Class 12 Solutions |
| CBSE Class 12 Full Study Material | CBSE Class 12 Sample Paper 2024-25 |
32. Arrange the following compound groups in the increasing order of their property indicated:
Propanol, Propane, Propanal (boiling point) (2017 Delhi)
Answer ⇒ Propanol > Propanal > Propane (Boiling point)
33. Formaldehyde does not take part in Aldolcondensation. Why? (2012 Comptt. All India)
Answer ⇒ Formaldehyde does not contain α-hydrogenatom. Therefore it does not take part in aldolcondensation.
34. Aldehydes and Ketones have lower boiling points than corresponding alcohols. Why? (2012 Comptt. All India)
Answer ⇒ It is due to weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
35. Give a chemical test to distinguish between Propanal and Propanone. (2012 Comptt. Delhi)
Answer ⇒ Propanone on reacting with I2 and NaOH gives a yellow ppt of iodoform but propanaldoes not respond to this test.
36. Give a chemical test to distinguish between Benzoic acid and Phenol. (2012 All India)
Answer ⇒ Benzoic acid forms a brisk effervescence with NaHCO3 solution but phenol does not respond to this test.
37. Aromatic primary amines cannot be prepared by Gabriel's phthalimide synthesis. Give reason. (2020 Series: HMJ/5)
Answer ⇒ Aromatic primary amines cannot be prepared by Gabriel's phthalimide synthesis as aryl halides do not undergo nucleophilic substitution reaction with the potassium salt formed by reaction between phthalimide and ethanolic potassium hydroxide.
38. Explain why (CH3)2NH is more basic than (CH3)3N in aqueous solution. (2023 Series: HFG1E/5)
Answer ⇒ Secondary amines (CH3)2NH are more basic than tertiary amines (CH3)3N due to steric hindrance of 3 bulky groups in 3° amine, which hinders the protonation at the nitrogen atom and hence reduces its basicity.
39. Aromatic diazonium salts are more stable than aliphatic diazonium salts. Give reason. (2018)
Answer ⇒ Aromatic diazonium salts are more stable than aliphatic diazonium salts because aromatic salts can be stabilized by dispersal of positive charge over the benzene ring through resonance, while alkyldiazonium salts readily decompose to form carbocation and nitrogen gas.
40. What type of protein is present in Keratin? (2020 Series: HMJ/4)
Answer ⇒ Fibrous protein is present in Keratin.
41. What is the effect of denaturation on the structure of proteins? (2020 Series: HMJ/5)
Answer ⇒ Due to denaturation, the secondary and tertiary structures of protein are destroyed as their globules get unfolded and helixes get uncoiled and thus enzyme loses its activity. However, primary structure of protein remains unaffected.
42. What is the difference between native protein and denatured protein? (2019 Series: BVM/4)
Answer ⇒ Native protein is the protein which is found in a biological system having a unique three-dimensional structure and specific biological activity.
43. Define the Fibrous protein term with a suitable example. (2018)
Answer ⇒ Fibrous protein :- These are linear thread-like molecules which tend to lie side by side to form fibres and their polypeptide chains are held together by hydrogen and disulphide bonds. For example, Keratin in skin, hair, etc.
👉 Read Also - CBSE 12th Chemistry Exam 2025 : Important Competency Based Questions with Answers for Last Minute Revision
44. Give one example each of (i) Globular protein, (ii) Fibrous protein (2013 Comptt. All India)
Answer ⇒
(i) Globular protein: All enzymes and hormones like insulin.
(ii) Fibrous protein: Keratin in skin, nails, etc.
45. State two functions of carbohydrates. (2012 Comptt. All India)
Answer ⇒ Two functions of carbohydrates:
(i) Carbohydrates such as glucose, starch, glycogen etc. provide energy for functioning of living organisms.
(ii) Carbohydrates, especially cellulose in the form of wood is used for making furniture, houses etc. by us.
46. The two strands in DNA are complimentary to each other. Give reason. (2023 Series: HFG1E/5)
Answer ⇒ DNA has a double helical structure, containing two strands which are held together by hydrogen bonds between specific pairs of bases. Cytosine forms a hydrogen bond with guanine, while adenine forms an H-bond with thymine. Thus, the sequence of bases in one strand automatically determines that of the other. Hence, the two strands of DNA are complementary to each other.
47. What are the hydrolysis products of DNA? (2020 Series: HM/5)
Answer ⇒ Hydrolysis products of DNA are a pentose sugar, i.e., 2-deoxy-D-ribose, phosphoric acid, and nitrogenous bases like Adenine, Guanine, Cytosine, and Thymine.
48. What type of linkage is present in Nucleic acids? (2016 All India)
Answer ⇒ Phosphodiester linkages are present in Nucleic Acids.
49. Why Vitamin C cannot be stored in our body? (2016 Delhi)
Answer ⇒ Vitamin C is mainly ascorbic acid, which is water-soluble and is readily excreted through urine and thus cannot be stored in the body.
50. Give reasons for the following observations: (2023 Series: HFG1E/5)
Water-soluble vitamins must be taken regularly in diet.
Answer ⇒ Water-soluble vitamins dissolve in water and are not stored by the body. Moreover, they are eliminated from the body in the form of urine, so we require a continuous supply of these vitamins in our diet.
👉 Download PDF - CBSE 12th Chemistry Exam 2025 : Important Very Short Questions with Answers







 Profile
Profile Signout
Signout








 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










