CBSE 12th Chemistry Exam 2025 : Important Competency Based Questions with Answers for Last Minute Revision

SHARING IS CARING
If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
CBSE 12th exams are underway and your CBSE 12th Chemistry exam is scheduled on 27th Feb, 2025. You have just a few hours left for CBSE 12th Chemistry exam.
We know how important it is to focus on the right topics and questions during revision so this article provides details of important CBSE 12th Chemistry Competency-Based questions along with answers for last minute revision.
Here, CBSE Class 12 Chemistry CBQs for all included chapters are provided in free PDF. Students are advised to solve these questions before the exam so that they are 100% prepared to solve questions from the actual board paper.
👉 Read Also - CBSE 12 Chemistry Exam 2025 : Top 50 MCQs with Answers for Last-Minute Revision - Download PDF
CBSE Class 12 Chemistry Competency-Based Questions 2025
Around 50% of the questions will be CBQs. So, prepare for them from the given below for Class 12 Chemistry.
1. A glycerine solution, at 293 K, has a molality of 3.89 molal and molarity of 5.33 M.
Which of these would be correct for molarity and molality of the same glycerine solution at 450 K?
(A) Molarity< 5.33 M; Molality 3.89 molal
(B) Molarity < 5.33 M; Molality < 3.89 molal
(C) Molarity > 5.33 M; Molality= 3.89 molal
(D) Molarity 5.33 M; Molality= 3.89 molal
Ans. (A)
2. In a chemistry laboratory, a student has 0.01 L of 102 mol dm3sulphuric acid.solution. The lab assistant asked the student to reduce its concentration to 2 x 10 mol dm³ by adding water into it. What should be the volume of the water added?
(A) 200 mL
(B) 490 mL
(C) 500 mL
(D) 510 mL
Ans. (B)
👉 Read Also - CBSE 12th Chemistry Exam 2025 : Most Important Assertion Reason Questions with Answers for Last Minute Revision
3. Solubility of gases in liquids decreases with rise in temperature because dissolution is an
(A) endothermic and reversible process
(B) exothermic and reversible process
(C) endothermic and irreversible process
(D) exothermic and irreversible process
Ans. (B)
4. A mixture of acetone and chloroform forms a maximum boiling azeotrope at a specific Which of these is correct for the mixture?' composition.
(A) Change in volume on mixing will be positive.
(B) Change in enthalpy on mixing will be positive.
(C) Interaction between unlike molecules is stronger than that between like molecules in the mixture.
(D) The proportion of acetone and chloroform in the mixture in the liquid phase is not the same as in the vapour phase.
Ans. (C)
👉 Read Also - CBSE Class 12 Chemistry 2025: Chapter-Wise Competency-Based Questions with Solutions – Free PDF Download
5. Which of the following should be done to change 100 mL of 0.1 M KCl solution to 0.2 M?
I. Reduce volume of solution to half by evaporation
II. Add 50 mL water
III. Add 0.1 mol KCl
IV. Add 0.01 mol KCI
(A) I and III
(B) I and IV
(C) II and IV
(D) Any of I, II, III and IV sid
Ans. (b)
6. How much electricity in Faraday is required for the complete reduction of MnO4 ions present in 500 mL of 0.5 M solution to Mn 2+?
(A) 5 F
(B) 2.5 F
(C) 2.25 F
(D) 1.25 F
Ans. (D)
7. Copper metal is purified by electrolytic refining. If the electrolyte used for refining of copper in an electrolytic cell is aqueous salt solution of copper, which out of the following statement about this cell is incorrect?
(A) The impure copper rod undergoes oxidation.
(B) Oxidation takes place at the anode.
(C) Impure copper rod acts as the negative electrode.
(D) Pure copper rod acts as a cathode.
Ans. (C)
8. An electrolytic cell has an anode and cathode made up of graphite. At the anode, Cl2 gas is released and at the cathode, H₂ gas is released. Which of the following electrolytes in the cell can produce these gases?
(A) NH4Cl(aq)
(C) NaCl (aq)
(B) Molten NH4Cl
(D) Molten NaCl
Ans. (C)
9. The electrochemical cell made up of Zn and Cu half-cell is called Daniell cell. The emf of a Daniell cell is 1.10 V.
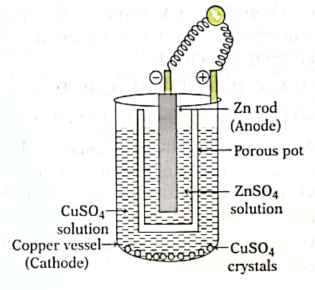
When an external voltage greater than 1.10 V is applied to this cell, which of the following change will be observed in the cell?
(A) Zn electrode will act as an anode.
(B) Current will flow from Cu half cell to Zn half cell.
(C) Electrochemical cell continue to work fast.
(D) Cell will act as electrolytic cell.
Ans. (D)
10. There are two beakers 'A' and 'B' containing KCl and CH3COOH solutions respectively. On adding water to beakers A and B, which of the following change in A, of the solutions will be correct?
(A) It increases sharply in beaker A and slowly in beaker B.
(B) It increases slowly in beaker A and sharply in beaker B.
(C) It decreases in beaker A but no change in bekaer B.
(D) There is no change in beaker A but it decreases slowly in beaker B.
Ans. (B)
👉 Read Also - CBSE Class 12 Chemistry Exam 2025: Important Questions, PYQs & Sample Papers for All Chapters - Free PDF Download
11. Which of the following statements is/are correct?
I. A catalyst lowers the activation energy of a reaction.
II. A catalyst allows the same rate of reaction to be achieved at a lower temperature.
III. A catalyst mixes with the reactants and increases the overall concentration of reactants in the rate equation.
(A) Only I
(B) I and II
(C) II and III
(D) All I, II and III
Ans. (B)
12. Which of the following can increase the rate of a chemical reaction?
I. Increasing the temperature.
II. Increasing the concentration of products.
III. Adding a catalyst.
IV. Increasing the concentration of reactants.
(B) I and II
(A) II and IV
(C) I, III and IV
(D) All I, II, III and IV
Ans. (C)
13. Which of the following is the unit of the rate constant for a zero order reaction?
(A) s-¹
(B) mol L-¹
(C) mol L-' s-¹
(D) L mol-¹ s-¹
Ans. (c)
14. Which of the following graphs represents a zero order rate of reaction?
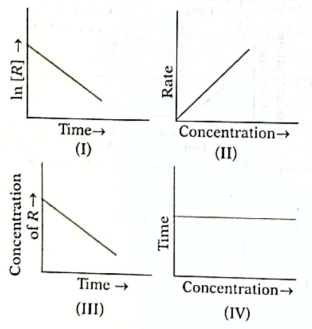
(A) I
(C) III
(B) II
(D) IV
Ans. (C)
15. Which of the following pairs of graphs represents the same order of reaction?
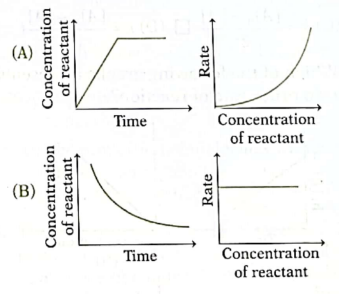
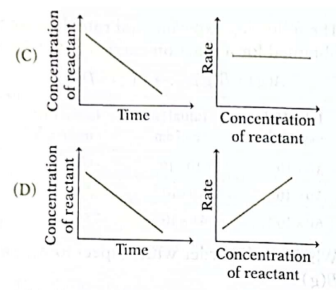
Ans. (C)
16. How many ions will be produced by the complex compound [Cr (en), ]Cl3, when it is dissolved in water?
(A) 2
(C) 7
(B) 4
(D) 10
Ans. (B)
👉 Read Also - CBSE Board Class 12 Chemistry Exam 2025 : Chapter-Wise Most Predicted Questions with Answers; Download Free PDF
17. When a co-ordination compound is dissolved in water it produces three moles of potassium ion as cation and one mole co-ordination entity as anion.The central metal ion Fe in entity is surrounded by three didentate anionic ligands. What is the oxidation state of Fe ion in the compound?
(A) 2
(B) 3
(C) 0
(D) 1
Ans. (B)
18. A co-ordination compound pentaaminechloridocobalt(III) sulphate is dissolved in water. When a few drops of chemical 'A' is added to the solution, it gives white precipitate. Identify chemical 'A'.
(A) AgCl
(B) AgNO3
(C) BaSO 4
(D) BaCl2
Ans. (D)
19. [M(AA)X2Y2] is a type of a co-ordinate compound in which M = metal ion, AA = didentate ligand, X = monodentate ligand, and Y = monodentate ligand. Which of the following isomerisms does this compound exhibit?
(A) Co-ordination isomerism
(B) Linkage isomerism
(C) Geometrical isomerism
(D) Optical isomerism
Ans. (D)
20. Which of the following coordination compounds is diamagnetic, has zero unpaired electrons and has an octahedral geometry? [Atomic number: Mn=25, Ni=28, Fe=26, Cu=29]
(A) [MnCl6]3-
(B) [Ni(CN4)]2-
(C) [Fe(CN)6]4-
(D) [CuCl4]²-
Ans. (C)
21. As per the crystal field theory, which of the following is correct about the repulsion between ligands and dy, dy, d orbitals in tetrahedral complexes?
(A) It is more than that in octahedral complexes
(B) It is less than that in octahedral complexes.
(C) It is the same as in octahedral complexes
(D) It is zero.
Ans. (A)
22. In an octahedral coordination entity the metal ion is surrounded by 6F ions. If crystal field splitting energy for this complex is ∆o and electron pairing energy is P then which of the following expression is correct about the complex?
(A) Ao = P
(B) Ao <P
(C) Ao >P
(D) Ao ≥ P
Ans. (B)
👉 Read Also - CBSE Class 12 Chemistry Board Exam 2025 : Most Repeated Questions from Last 10 Years; Download PDF
23. Which of the following molecules exhibits optical isomerism?
(A) 3-iodopentane
(B) 2-iodo-2-methylpropane
(C) 1, 3-diiodopropane
(D) 2-iodobutane
Ans. (D)
24. Observe the given reaction.
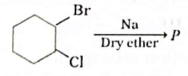
Which of the following products will be formed as P?
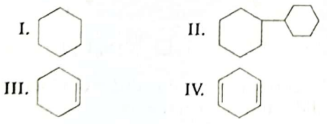
(A) I
(B) II
(C) III
(D)IV
Ans. (C)
25. The table given below shown some of the features of S1 and S2 reaction mechanism.
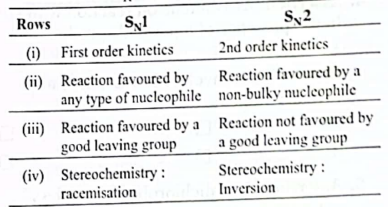
Which of the rows shows an incorrect feature for at least one of the mechanisms?
(A) (i)
(B) (ii)
(C) (iii)
(D) (iv)
Ans. (c)
26. In which of these compounds is the lengthof the carbon-oxygen bond the shortest?
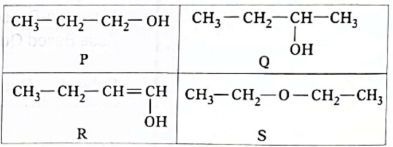
(A) P
(B) Q
(C) R
(D) S
Ans. (C)
27. The boiling points of four compounds, an ether, an aldehyde, an alcohol, and a haloalkane of comparable molecular weights, are given (not necessarily in the same order) in the table below.
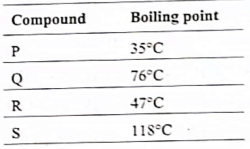
Identify, which of the four compounds is the alcohol?
(A) P
(C) R
(B) Q
(D) S
Ans. (D)
28. Given below are four examples in which thereactants and the reactions they are subjected to are stated.
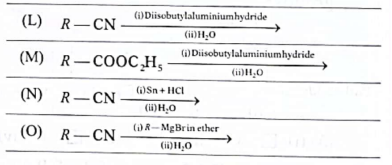
Identify the example(s) in which the major product obtained will be an aldehyde and inwhich example(s) it is a ketone.

(A) (a)
(B) (b)
(C) (c)
(D) (d)
Ans. (D)
👉 CBSE Class 12 Study Materials
| CBSE Class 12 Syllabus 2024-25 | CBSE Class 12 Previous Year Papers |
| NCERT Books For Class 12 Books | NCERT Class 12 Solutions |
| CBSE Class 12 Full Study Material | CBSE Class 12 Sample Paper 2024-25 |
29. Electrophilic substitution in benzoic acid takes place at the meta-position. Which of the following is the reason for the reaction above?
(A) The carboxyl group activates only the meta-position.
(B) The carboxyl group deactivates only the ortho and para-positions.
(C) The carboxyl group activates the meta-position more than the ortho and para-positions.
(D) The carboxyl group deactivates the meta-position less than the ortho and para-positions.
Ans. (D)
30. A carbonyl compound X does not give a reddish-brown precipitate on heating with Fehling's solution.
Which of the following could compound X be?
I. Propanal II. Diethylketone III. 4-nitrobenzaldehyde
(A) (III) and
(IV) (B) Only
(II) (C) either
(I) or (III)
(D) either (II) or (III)
Ans. (D)
31. A carbonyl compound produces iodoformon reaction with sodium hypoiodite. Which of the following could the carbonylcompound be?
I. CH3 -CH₂- CHО
II. CH3 -CH₂-CO—CH2- CH3
III. CH3 - CHO IV. CH3 -CH₂-- Со - CH3
(A) Only (I)
(B) (I) and (III)
(C) (II) and
(IV) (D) (III) and (IV)
Ans. (D)
32. A carbonyl compound X undergoes the reactions given in the table below.

of the following could compound X Which be?
(A) CH3-CH₂-CHО
(B) CH3-CO-CH3
(C) CH3-СНО
(D) H-CHO
Ans. (C)
33. Which of the options correctly identifies theamount of ammonia and alkyl halide usedin the reaction and the type of amine obtained?
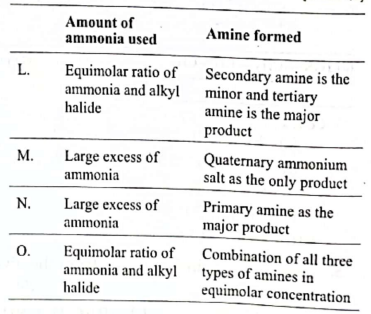
(A) L
(B) M
(C) N
(D) O
Ans. (C)
34. The graphs below show the solubility of a primary, a secondary and a tertiary aliphatic amine I, J and K in water, at the same temperature. The number of carbon atoms in each of the compounds is three. Amine I is the tertiary amine, amine J is the primary amine and amine K is the secondary amine.
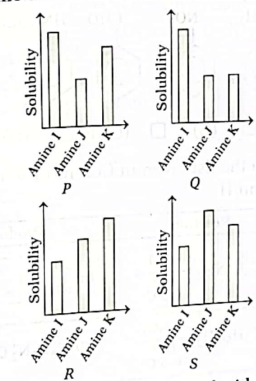
Which of the following graphs identifies the three amines correctly?
(A) P
(B) Q
(C) R
(D) S
Ans. (D)
35. Which of the following is true about the solubility of ethylamine and aniline? (CBSE QB]
(A) Aniline is soluble in HCl.
(B) Both are insoluble in HCl.
(C) Both are soluble in water.
(D) Ethylamine is insoluble in water.
Ans. (A)
36. Two isomers, n-C4H,NH₂ and (C₂H3)2NH have molar mass of 73 each. Which of the following statement is correct about their boiling points?
(A) The boiling point of n-C4H,NH2 is higher than that of (C₂H3)2NH.
(B) The boiling point of (C2H3)2NH is higher than that of n- C4H9NH2.
(C) Both the amines will have the same boiling point.
(D) The boiling point of both the amines will be lower than that of water.
Ans. (A)
37. The same volume of three isomeric amines are boiled and the time taken for vapourisation of the entire volume is noted in the table given below.

Which of the following statement is most likely to be true about these three amines?
(A) The expected molar mass of amine G and H are different.
(B) Amine F is most likely to be a primary amine.
(C) Amine G is most likely to be a secondary amine.
(D) The expected molar mass of amine F is greater than that of amine H.
Ans. (C)
38. Aniline on heating with chloroform and alcoholic KOH gives a foul-smelling product. Making which of the following changes in the reaction would still produce a foul-smelling product?
(P) Replacing aniline with ethylamine
(Q) Replacing chloroform with carbon tetrachloride
(R) Replacing alcoholic KOH with alcoholic NaOH
(A) Only P
(B) Only R
(C) Only Q and R
(D) Only P and R
Ans. (D)
39. Benzene sulphonyl chloride is a chemical, which can be used to identify the class of an Amine. When an amine 'A' reacts with benzene sulphonyl chloride it gives precipitate of sulphonamides, which is soluble in alkali. The amine A is
(A) N-Ethylethanamine
(B) N,N-Diethylethanamine
(C) Ethanamine
(D) N-methylbenzenenamine
Ans. (C)
40. Aryl diazonium salts undergo reductive removal of the diazonium group in presence of weak acids. Which of the following products will be formed during this process?
(A) Chlorobenzene
(B) Phenol
(C) Benzene cyanide
(D) Benzene
Ans. (D)
41. The reaction of an arene diazonium chloride with aniline in an acidic medium gives a coloured compound. Which of the following occurs during the reaction?
(A) Benzene ring is replaced.
(B) Nitrogen is displaced.
(C) Diazo group is retained.
(D) Amino group is displaced.
Ans. (C)
42. Shown below is the chain structure of an unknown compound A.
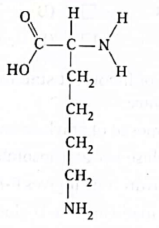
Which of the following statements is true for compound A?
(A) Compound A is neutral.
(B) Compound A is basic in nature.
(C) Compound A is acidic in nature.
(D) Compound A is ammonium salt.
Ans. (B)
43. Which is the structure of a zwitter ion of an amino acid?
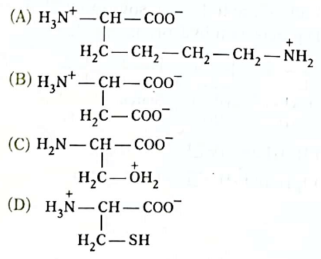
Ans. (D)
44. Which of the following statements is/are correct?
I. Amongst lysine, histidine and Serine, lysine is the most basic in nature.
II. All non-essential amino acids are basic in nature.
III. Adding acids such as lemon juice into meat protein does not denature the primary structure yet tenderize meat.
(A) Only I
(B) Only III
(C) I and III
(D) I, II, and III
Ans. (B)
45. Which of the following statements is/are correct for proteins or enzymes when they are subjected to physical changes as specified?
I. The sequence of amino acids in the peptide changes in a protein when the pH of its environment is changed.
II. Most enzymes stop working above about 50°C.
III. Albumin, a globular protein found in egg whites, sets into an insoluble white solid when the egg white is heated.
(A) Only III
(C) II and III
(B) I and II
(D) I, II and III
Ans. (C)
46. The following image shows the structure of DNA, with the letters indicating the bases present.
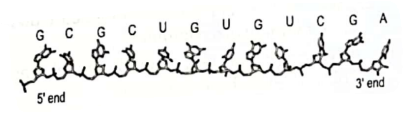
Which structure of DNA is represented above?
(A) Primary
(B) Secondary
(C) Tertiary
(D) Quaternary
Ans. (A)
47. 342.3 g of sucrose is dissolved in 1 kg of water in a pot to form a solution. The boiling point of water (solvent) is 373.15 K. Which of the following is likely to be the boiling point of the solution? (Molar mass of sucrose = 342.3 g/mol; Atmospheric pressure = 1.013 bar; K=0.52 K kg mol-')
(A) 373 K
(B) 373.15 K
(C) 373.67 K
(D) 372.63 K
Ans. (C)
48. Which of the following statement is/are true?
I. The freezing point of 0.1 M KCl is higher than that of 0.1 M C₂H,OH.
II. The freezing point of a 4% aqueous solution of X having molecular weight as m is equal to the freezing point of 12% aqueous solution of Y having molecular weight 3m (assume that i = 1 for both X and Y).
III. The boiling point of pure water at sea level is greater than at mount Everest.
(A) I and II
(C) II and III
(B) I, II and III
(D) I and III
Ans. (C)







 Profile
Profile Signout
Signout












 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










