CBSE Class 10 Science 2024-25: Chapter 2 Acids, Bases and Salts Competency-Based Questions with Answers; Download Free PDF

SHARING IS CARING
If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
As the CBSE Class 10 board exams get closer, it’s important for students to understand the new exam pattern. Starting in the 2024-25 school year, CBSE will include 50% more competency-based questions. These questions will be both multiple choice and written, focusing on how to use what students have learned in real-life situations.
This article explores Chapter 2: Acids, Bases and Salts. It highlights key competency-based questions and provides answers to help students succeed. Prepare for your CBSE Class 10 Science exams with important competency-based questions from Chapter 2: Acids, Bases and Salts.
Understanding Competency-Based Questions in Chapter 2: Acids, Bases and Salts
Get ready for the CBSE Class 10 Science 2024-25 exams with key competency-based questions from Chapter 2: Acids, Bases and Salts. These questions help you apply concepts in real-life situations. Download the free PDF for detailed answers and boost your exam preparation.
CBSE Class 10 Science Chapter 2: Acids, Bases and Salts Important Competency-Based Questions
Q.1 Hydrangea plants develop blue or pink flowers depending on the availability of aluminium from the soil. When the soil is acidic, aluminium is more available to the roots, resulting in blue flowers. When the soil is alkaline, the availability of aluminium decreases, resulting in pink flowers.
The graph below is of the pH of the soil at different sections of a field.
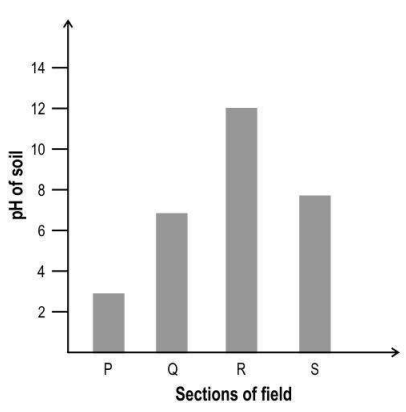
In which section of the field will the flowers on ALL the hydrangea plants definitely be blue in colour and in which section will the flowers on ALL the hydrangea plants definitely be pink in colour?
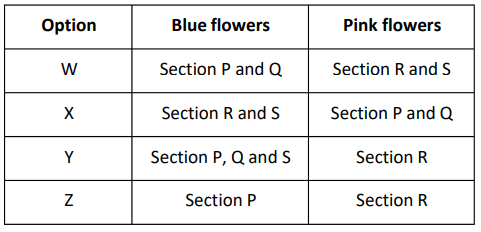
A. W
B. X
C. Y
D. Z
Answer. (D). Z
Q: 2 Adding which of the following to a colourless solution would give an indication that the solution could possibly be hydrochloric acid?
1. copper metal strips
2. silver metal strips
3. calcium carbonate
4. sodium chloride
Answer. (3)
| Download PDF | |
| CBSE Class 10 Science Chapter 1 Chemical Reactions & Equation: Important Competency-Based Questions 2024-25 | Click Here |
| CBSE Class 10 Science Chapter 2 Acids, Bases and Salts: Important Competency-Based Questions 2024-25 | Click Here |
Q: 3 Which of these graphs shows how the pH of milk changes as it forms curd?
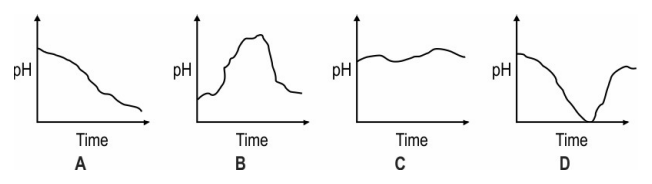
Answer. (1)
Q.4 The presence of acidic gases in the air increases the rate of corrosion. Furthermore, an increase in temperature can also increase the rate of corrosion.
The graph below is created under 4 different conditions (shown below in the table) of temperature and acidic nature of air.
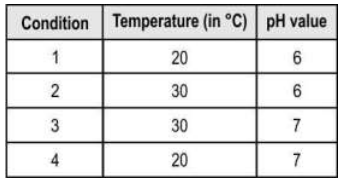
Which of the graphs represents condition 2?
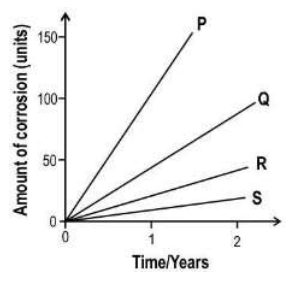
(a). P
(b). Q
(c). R
(d). S
Answer. Graph P
Q.5 An excess of carbon dioxide gas is bubbled through lime water.
(a) Will the pH of lime water change? If yes, how? Explain your answer.
(b) Write the balanced equation for the reaction.
Answer. (a) - The pH of lime water will decrease. [0.5 marks]
- Carbon dioxide, being an acidic oxide, will neutralise lime water which is basic. [1 mark]
OR
-The products formed, namely, calcium carbonate and Calcium hydrogen carbonate are basic salts butless basic than Calcium hydroxide so pH decreases.
(b) 0.5marks each for writing the formula of lime water and the product calcium bicarbonate; 0.5 marks for balancing the equation:
Ca(OH)2 + 2 CO2 ---> Ca(HCO3)2
OR
Ca(OH)2 + CO2 ---> CaCO3 + H2O
CaCO3 + CO2 + H2O ---> Ca(HCO3)2
Q.6 Tanu takes 500 mL milk each in two bowls P and Q. She adds curd to both the bowls and baking soda only to bowl Q as shown below.
(a) Bowl P - 500 mL milk + 1 teaspoon curd
(b) Bowl Q - 500 mL milk + 1 teaspoon curd + 1 teaspoon baking soda
In which bowl will the milk form into curd faster? Explain your answer.
Answer. The milk will form into curd faster in bowl P.
- Curdling ofmilk takes place due to formation of lactic acid by bacteria. [1mark]
- In bowl Q, the lactic acid formed by bacteria has to first neutralise the baking soda, which is basic in nature, before the milk starts curdling. [1 mark]
Q.7 A solution P is taken in a flask and two drops of phenolphthalein indicator is added to it. The graph below shows how the pH of the mixture changes as a solution Q is added dropwise to the flask with stirring.
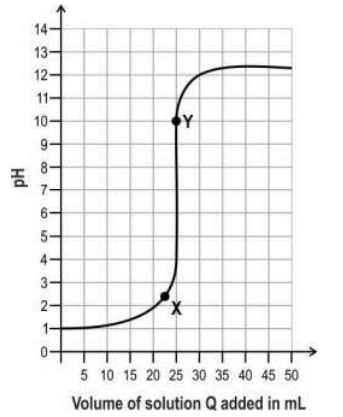
(a) Identify the nature of solutions P and Q.
(b) What will the colour of the solution in the flask be at points X and Y?
(c) Identify the type of reaction taking place in the flask.
(a) 0.5 marks each for the following:
- solution P: acidic
- solution Q: basic
(b) 0.5 marks for each of the following:
- colour of solution at X: colourless
- colour of solution at Y: pink
(c) neutralisation
Q.8 Aditi adds 1 mole of dilute hydrochloric acid to an aqueous solution of 1 mole of sodium carbonate.
(a) Write the balanced equation for the reaction that takes place.
(b) How will the colour of a red litmus and a blue litmus paper change when dipped in this mixture? Explain why.
Answer. (a) 0.5marks each for writing the formula of each reactant and product:
HCl + Na2CO3 ---> NaCl + NaHCO3
(b)
- Red litmus paper will turn blue. [0.5 marks]
- There will be no effect on blue litmus paper. [0.5 marks]
- The complete neutralisation of 1 mole of sodium carbonate requires 2 moles of hydrochloric acid. [1 mark]
- Since only 1 mole of HCl is used, neutralisation is incomplete and the mixture will be basic. [1 mark]
Q: 9 To prepare a salad dressing, Parag adds a solution of sodium chloride in distilled water to vinegar.
State what change will occur in the following:
(i) the pH of the vinegar
(ii) the acidity of the vinegar
Rajesh was given a substance and asked to identify it. He conducted three tests on the substance and recorded the results below. (P) It releases carbon dioxide, water and a sodium salt on heating with water.(Q) It turns universal indicator greenish-blue.(R) It can be prepared from ammonia as a raw material.
Answer. (i) The pH will increase.
(ii) The acidity will decrease.
Q: 10 What substance was Rajesh given?
Answer. baking soda / sodium hydrogencarbonate / NaHCO3
Q: 11 Give ONE use of the substance based on the properties mentioned in P and Q.
Answer. 1 mark for any of the following:
- used in antacids
- used in toothpaste
- used as a first aid in acidic insect bites
Q: 12 Rajesh later read that recrystallisation of the sodium salt formed in P gives another basic salt that is used in manufacture of borax.
Identify the sodium salt formed in P.
Answer. sodium carbonate / Na, CO,3
Q: 13 Aditi finds that a mixture of an acid and a base does not change the colour of either red or blue litmus paper.
Compare the amounts of H+ and OH in the solution.
Answer. The amount of H* is equal to the amount of OH in the solution.
Q: 14 pH is measured on a scale of 0 to 14, with lower values indicating high hydrogen ion concentration (more acidic) and higher values indicating low hydrogen ion concentration (less acidic). A pH of 7 is considered as neutral. Every whole unit in pH represents a ten-fold increase in or decrease in hydrogen ion concentration. What would the hydrogen ion concentration of a solution of pH 4 be compared to a solution of pH 8?
Answer. A solution of pH 4 would have 10,000 times higher concentration of hydrogen ions compared to a solution of pH 8.
Q: 15 pH is measured using a pH meter, which comprises a detecting unit consisting of a pH sensitive glass electrode and an indicating unit which indicates the pH as shown below.

To measure the pH of a solution, the glass electrode is dipped into the solution and the pH is displayed on the screen of the indicating unit. Before measuring the pH of another solution, the glass electrode is rinsed with distilled water and dried carefully with tissue paper.
How is the pH reading of the second solution likely to be affected if the glass electrode is not dried with tissue paper in the following cases?
(i) if the second solution being measured is acidic in nature
(ii) if the second solution being measured is basic in nature
Answer. (i) The pH meter will indicate a slightly higher pH reading than the actual pH of the solution if the second solution is acidic.
(ii) The pH meter will indicate a slightly lower pH reading than the actual pH of the solution if the second solution is basic.
-
👉 Read Also- CBSE Class 10 Half-Yearly/Mid Term 2024-25 : Most Important Questions with Answers; PDF Download (All Subjects)
👉 CBSE Class 10 Study Materials
| CBSE Class 10 Syllabus 2024-25 | NCERT Solutions For Class 10 |
| CBSE Class 10 Previous Year Question Papers | CBSE Class 10 Books |
| CBSE Class 10 Full Study Material | CBSE Class 10 Sample Paper |







 Profile
Profile Signout
Signout












 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










