CBSE 12th exams are underway and your CBSE 12th Chemistry exam is scheduled on 27th Feb, 2025. You have just a few hours left for CBSE 12th Chemistry exam.
We know how important it is to focus on the right topics and questions during revision, so this article provides important CBSE 12th Chemistry Long Type Questions along with Answers for last minute revision.
👉 Read Also - CBSE 12 Chemistry Exam 2025 : Top 50 MCQs with Answers for Last-Minute Revision - Download PDF
Scroll down, full question and answer PDF is given. Get most repeated questions for CBSE Class 12 Chemistry. These questions are important and often appear in exams. Practice them well to prepare for the CBSE Class 12 Chemistry Exam 2025.
👉 Read Also - CBSE 12th Chemistry Exam 2025 : Important Very Short Questions with Answers for Last Minute Revision
Important Long Type Questions in CBSE Class 12 Chemistry Exam 2025
1. The elements of 3d transition series are given as:
Sc Ti V Cr Mn Fe Co Ni Cu Zn
Answer the following:
(i) Write the element which shows maximum number of oxidation states. Give reason.
(ii) Which element has the highest m.p.?
(iii) Which element shows only +3 oxidation state?
(iv) Which element is a strong oxidizing agent in +3 oxidation state and why? (2016 All India)
Answer ⇒
(i) Mn shows maximum number of oxidation states up to +7. It has the maximum number of unpaired electrons.
(ii) Cr has the highest melting point.
(iii) Sc shows only +3 oxidation state.
(iv) Mn is a strong oxidizing agent in +3 oxidation state because after reduction it attains +2 oxidation state in which it has the most stable half-filled (d5) configuration.
👉 Read Also - CBSE 12th Chemistry Exam 2025 : Most Important Assertion Reason Questions with Answers for Last Minute Revision
2. (a) How do you convert the following:
(i) Phenol to Anisole
(ii) Ethanol to Propan-2-ol
(b) Why phenol undergoes electrophilic substitution more easily than benzene?
(2019 BVM)
Answer ⇒
(a) (i) Phenol to Anisole:
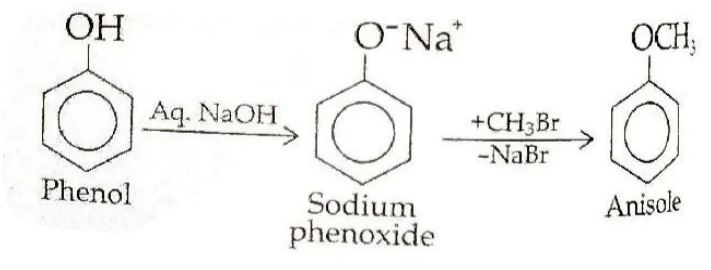
(ii) Ethanol to Propan-2-ol:
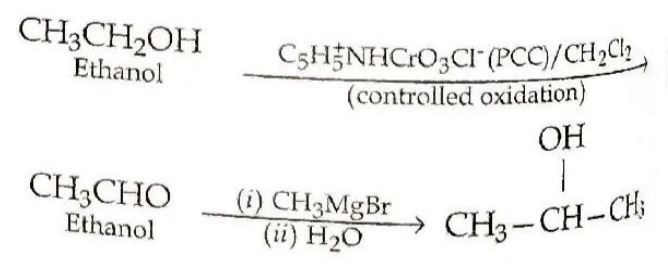
(b) Phenol undergoes electrophilic substitution more easily than benzene because of the strong activating effect of the –OH group attached to the benzene ring. The involvement of lone pair of oxygen in delocalisation makes the benzene ring electron rich.
👉 Read Also - CBSE Class 12 Chemistry 2025: Chapter-Wise Competency-Based Questions with Solutions – Free PDF Download
3. Give chemical tests to distinguish between the following pairs of compounds:
(i) Phenol and Benzoic acid
(ii) Propanal and Propanone (CBSE 2024)
Answer ⇒
(i) Phenol and Benzoic acid. Benzoic acid gives NaHCO3 test with the evolution of brisk effervescences of CO2 gas while phenol does not.
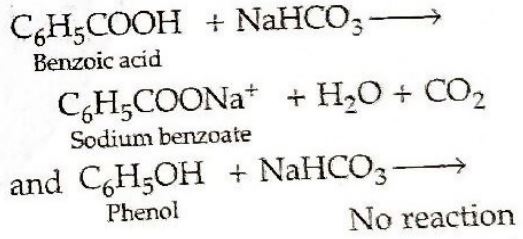
(ii) Propanal and Propanone. Propanalbeing an aldehyde gives positive testwith Fehling's solution in which a redbrown precipitate of Cuprous oxideis obtained while propanone being a
ketone does not respond to this test.
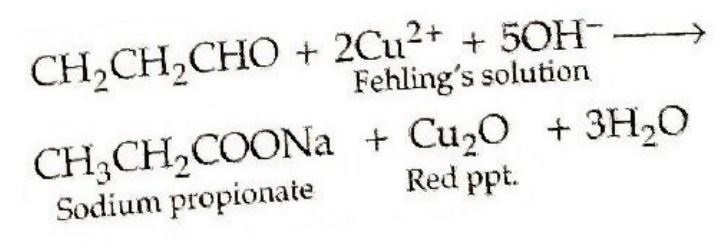
👉 Read Also - CBSE Class 12 Chemistry Exam 2025: Important Questions, PYQs & Sample Papers for All Chapters - Free PDF Download
4. Write the main structural difference between DNA and RNA. Of the four bases, name those which are common to both DNA and RNA of the two bases, thymine and uracil, which one is present in DNA? (2016 All India)
Answer ⇒
| DNA | RNA | |
|---|---|---|
| 1. | The sugar present in DNA is 2-deoxy-(-) ribose. | The sugar present in RNA is D-(-) ribose. |
| 2. | DNA contains cytosine and thymine as pyrimidine bases. | RNA contains cytosine and uracil as pyrimidine bases |
| 3. | DNA has double standard α-helixbases. | RNA has single stranded α-helix structure. |
| 4. | DNA molecules are very large. Their molecular mass may vary from 6 x 106 - 16 x 106 μ i.e., from six to sixteen million. | RNA molecules are much smaller with molecular mass ranging from 20,000 to 40,000 μ. |
👉 Read Also - CBSE Board Class 12 Chemistry Exam 2025 : Chapter-Wise Most Predicted Questions with Answers; Download Free PDF
5. Differentiate between fibrous proteins and globular proteins. What is meant by the denaturation of a protein?
Answer ⇒
| Globular Proteins | Fibrous Proteins | |
|---|---|---|
| 1. | Globular proteins have almost spheroidal shape due to folding of the polypeptide chain. | Polypeptide chains of fibrous proteins consist of thread like molecules which tend to lie side by side to form fibres. |
| 2. | Globular proteins are soluble in water. | Fibrous proteins are insoluble in water. |
| 3. | Globular proteins are sensitive to small changes of temperature and pH. Therefore they undergo denaturation on heating or on treatment with acids/bases. | Fibrous proteins are stable to moderate changes of temperature and pH. |
| 4. | They possess biological activity that’s why they act as enzymes. | They do not have any biological activity but serve as chief structural material of animal tissues. |
| Example: Maltase, invertase etc., hormones (insulin), antibodies, transport agents (haemoglobin), etc. | Example: Keratin in skin, hair, nails and wool, collagen in tendons, fibroin in silk, etc. |
Denaturation of protein: Due to coagulation of globular protein under the influence of change in temperature, change in pH etc., the native shape of the protein is destroyed and biological activity is lost and the protein so formed is called denatured proteins and the phenomenon is denaturation.
👉 Read Also - CBSE Class 12 Chemistry Board Exam 2025 : Most Repeated Questions from Last 10 Years; Download PDF
6. A 10% solution (by mass) of sucrose in water has a freezing point of 269.15 K. Calculate the freezing point of 10% glucose in water, if the freezing point of pure water is 273.15 K.
(2017 Delhi)
Given:
(Molar mass of sucrose = 342 g mol⁻¹)
(Molar mass of glucose = 180 g mol⁻¹)
Answer ⇒


👉 CBSE Class 12 Study Materials
| CBSE Class 12 Syllabus 2024-25 | CBSE Class 12 Previous Year Papers |
| NCERT Books For Class 12 Books | NCERT Class 12 Solutions |
| CBSE Class 12 Full Study Material | CBSE Class 12 Sample Paper 2024-25 |
7. Define molar conductivity of a substance and describe how for weak and strong electrolytes, molar conductivity changes with concentration of solute. How is such change explained? (2009 Delhi)
Answer ⇒ Molar conductivity: Molar conductivity of a solution at a given concentration is the conductance of the volume ‘V’ of a solution containing one mole of electrolyte kept between two electrodes with area of cross section ‘A’ and distance of unit length. It is represented by λm (lambda).
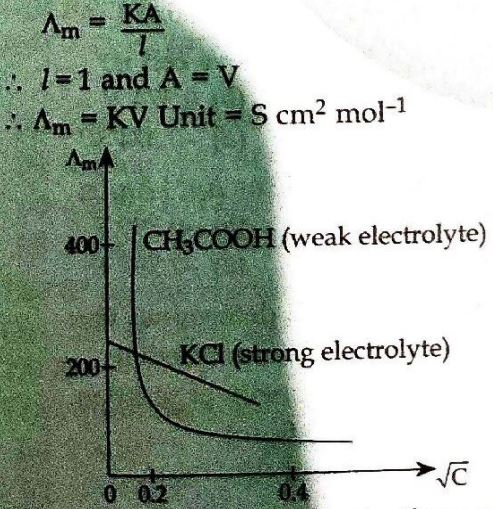
Effect of change of concentrations on molar conductivity. In case of strong electrolytes, there is a small increase in conductance with dilution because a strong electrolyte is completely dissociated in solution and the
number of ions remains constant. Moreover there will be greater inter-ionic attractions at higher concentrations which retards the motion of ions and conductance decreases. In case of weak electrolytes there is increase in conductance with decrease in concentration due to the increase in the number of ions in the solution.
The graph between λm and concentrationalso justifies the above statement.
👉 Read Also - CBSE 12th Chemistry Exam 2025 : Important Competency Based Questions with Answers for Last Minute Revision
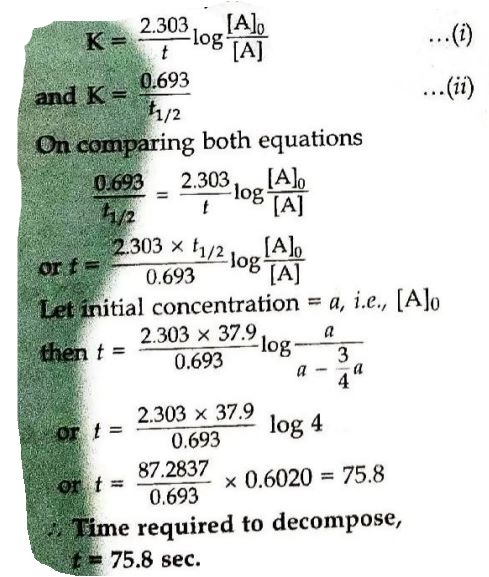
8. The decomposition of phosphine, PH₃, proceeds according to the following equation:
4 PH₃ (g) → P₄ (g) + 6 H₂ (g)
It is found that the reaction follows the following rate equation:
Rate = K [PH₃]
The half-life of PH₃ is 37.9 s at 120°C.
(i) How much time is required for 3/4th of PH₃ to decompose?
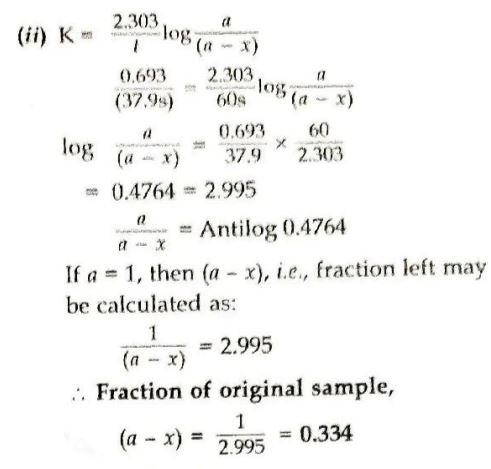
(ii) What fraction of the original sample of PH₃ remains behind after 1 minute?
Answer ⇒
(i) According to the formula:
👉 Read Also - CBSE 12th Chemistry Exam 2025: Important Short Type Questions with Answers for Last Minute Revision
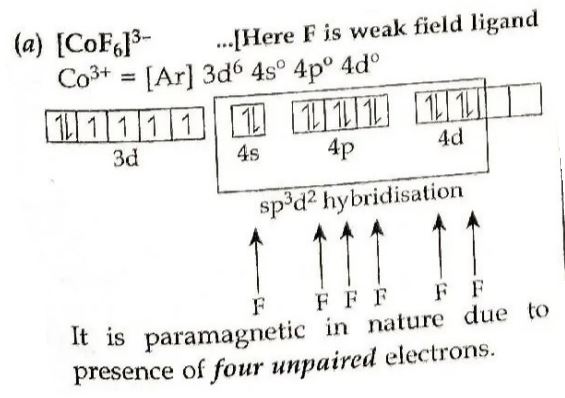
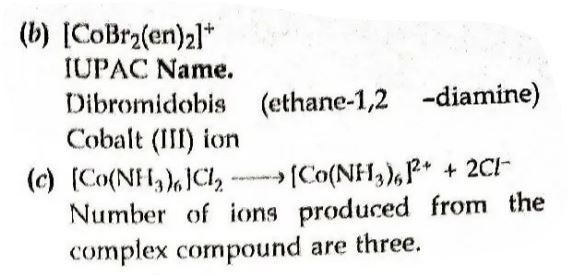
9. (i) Using valence bond theory, predict the hybridization and magnetic character of the following:
[CoF₆]³⁻ [Atomic number of Co = 27]
(b) Write IUPAC name of the following complex:
[CoBr₂(en)₂]⁺
(c) How many ions are produced from the complex [Co(NH₃)₆]Cl₂ in solution?
(2022 Term II)
Answer ⇒
10. How is the stability of a co-ordination compound in solution decided? How is the dissociation constant of a complex defined? (2012 Comptt. All India)
Answer ⇒ Stability of a complex in solution means the measure of resistance to the replacement of a ligand by some other ligand. This stability can be expressed in terms of equilibrium constant.
Let the reaction between metal and ligand be represented as
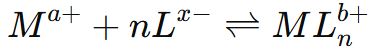
Stability or Dissociation constant (K)
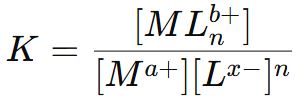
The reciprocal of the stability constant is known as instability constant or dissociation constant.
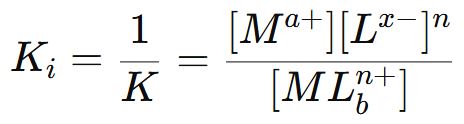
Factors affecting the stability of a complex ion
(i) Nature of metal ion: Greater the charge and smaller the size of the ion, more is its charge density and greater will be the stability of the complex.
(ii) Nature of ligand: More the basicity of ligand, more is its tendency to donate an electron pair and therefore, more is the stability of the complex.
Download CBSE Class 12 Chemistry 2025 Important Long Type Questions with Answers PDF. Strengthen your concepts and build confidence with these essential practice questions. Download now for FREE!







 Profile
Profile Signout
Signout








 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










