CBSE 12th Chemistry Exam 2025: Important Short Type Questions with Answers for Last Minute Revision

SHARING IS CARING
If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
CBSE 12th exams are underway and your CBSE 12th Chemistry exam is scheduled on 27th Feb, 2025. You have just a few hours left for CBSE 12th Chemistry exam.
We know how important it is to focus on the right topics and questions during revision, so this article provides important CBSE 12th Chemistry Short Type Questions along with Answers for last minute revision.
👉 Read Also - CBSE 12 Chemistry Exam 2025 : Top 50 MCQs with Answers for Last-Minute Revision - Download PDF
Scroll down, full question and answer PDF is given. Get most repeated questions for CBSE Class 12 Chemistry. These questions are important and often appear in exams. Practice them well to prepare for the CBSE Class 12 Chemistry Exam 2025.
👉 Read Also - CBSE 12th Chemistry Exam 2025 : Important Very Short Questions with Answers for Last Minute Revision
Important Short Type Questions in CBSE Class 12 Chemistry Exam 2025
1. State Henry’s law and mention two of its important applications. (2012 Comptt. All India, 2019 BVM/1)
Answer ⇒ Henry’s law: Henry’s law states that “The partial pressure of the gas in vapour phase is proportional to the mole fraction of the gas in the solution”.
Applications of Henry’s law:
(i) To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
(ii) To avoid a dangerous medical condition called bends, scuba divers use oxygen diluted with less soluble helium gas.
(iii) Deep sea divers depend upon compressed air for their oxygen supply.
👉 Read Also - CBSE 12th Chemistry Exam 2025 : Most Important Assertion Reason Questions with Answers for Last Minute Revision
2. State Faraday's first law of electrolysis. How much charge in terms of Faraday is required for the reduction of 1 mol of Cu2+ to Cu. (2014 Delhi)
Answer ⇒ According to first law of Faraday’s, “the amount of chemical reaction and hence the mass of any substance deposited/liberated at any electrode is directly proportional to the quantity of electricity passed through the electrolyte.”
The quantity of charge required for reduction of 1 mol of Cu2+ = 2 Faradays
(∵ Cu2+ + 2e- → Cu)
= 2 × 96500 C = 193000 C
3. Define electrochemical cell. What happens if external potential applied becomes greater than E⁰cell of electrochemical cell? (2016 All India, 2019 BVM/4)
Answer ⇒ Electrochemical cell: It is a device which converts chemical energy into electrical energy i.e., produced as a result of redox reaction taking place in the electrolyte.
If external potential applied becomes greater than E⁰ cell of electrochemical cell, the reaction gets reversed and it becomes non-spontaneous. It starts acting as an electrolytic cell.
👉 Read Also - CBSE Class 12 Chemistry 2025: Chapter-Wise Competency-Based Questions with Solutions – Free PDF Download
4. Define the term degree of dissociation. Write an expression that relates the molar conductivity of a weak electrolyte to its degree of dissociation. (2015 Comptt. Delhi)
Answer ⇒ Degree of dissociation: It is the measure of the extent to which an electrolyte gets dissociated into its constituent ions.
Thus, higher the degree of dissociation, higher will be its molar conductance.
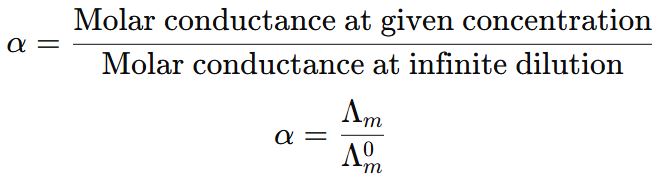
5. The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9 S cm2 mol-1. Calculate the conductivity of this solution. (2012 All India, 2016 Comptt. Delhi)
Answer ⇒ Given, C = 1.5 M, S cm2 mol-1
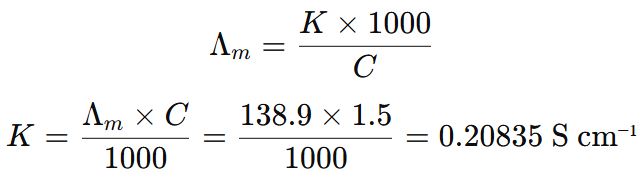
6. Write two differences between 'order of reaction' and 'molecularity of reaction'. (2014 Delhi)
Answer ⇒
| Order of reaction | Molecularity of reaction | |
|---|---|---|
| (i) | It is the sum of the concentration terms on which the rate of reaction actually depends. | It is the number of atoms, ions, or molecules that must collide with one another simultaneously so as to result into a chemical reaction. |
| (ii) | It can be fractional as well as zero. | It is always a whole number. |
7. For the hydrolysis of methyl acetate in aqueous solution, the following results were obtained:
| t/s | 0 | 30 | 60 |
|---|---|---|---|
| [CH3COOCH3] / mol L-1 | 0.60 | 0.30 | 0.15 |
Calculate the average rate of reaction between the time interval 30 to 60 seconds.
(Given log 2 = 0.3010, log 4 = 0.6021) (2015 Delhi)
Answer ⇒ Rate = -Δ[R] / Δt
Average rate between 30 to 60 seconds
= (0.15 - 0.30) / (60 - 30)
= (-0.15) / 30
= 0.5 × 10-2 mol L-1 sec-1
👉 Read Also - CBSE Class 12 Chemistry Exam 2025: Important Questions, PYQs & Sample Papers for All Chapters - Free PDF Download
8. What are the transition elements? Write two characteristics of the transition elements. (2015 Delhi)
Answer ⇒ Elements which have partially filled d-orbitals in their ground states or any one of its oxidation states are called transition elements.
(i) They show variable oxidation states.
(ii) They form coloured ions.
(iii) They form complex compounds.
9. Explain the following observations:
(i) Generally, there is an increase in density of elements from titanium (Z = 22) to copper (Z = 29) in the first series of transition elements.
(ii) Transition elements and their compounds are generally found to be good catalysts in chemical reactions.
(2010 Delhi, 2009 AI, 2011 AI, 2012 CD, 2013 D, 2014 CAI, 2019 BVM/1)
Answer ⇒
(i) From titanium to copper, the atomic size of elements decreases and mass increases, as a result of which density increases.
(ii) The catalytic properties of the transition elements are due to the presence of unpaired electrons in their incomplete d-orbitals and variable oxidation states.
👉 Read Also - CBSE Board Class 12 Chemistry Exam 2025 : Chapter-Wise Most Predicted Questions with Answers; Download Free PDF
10. Explain, giving a suitable reason for the following:
Metal-metal bonding is more frequent for the 4d and the 5d series of transition metals than that for the 3d series. (2011 All India)
Answer ⇒ Metal-metal bonding is more frequent for the 4d and the 5d series of transition metals than that for the 3d series as these have their electrons of the outermost shell at greater distance from the nucleus, as compared to atoms of 3d transition metals.
11. Assign a reason for each of the following observations:
(i) The transition metals (with the exception of Zn, Cd, and Hg) are hard and have high melting and boiling points.
(ii) The ionization enthalpies (first and second) in the first series of transition elements are found to vary irregularly. (2014 Comptt. Delhi)
Answer ⇒
(i) Because of stronger metallic bonding and high enthalpies of atomization.
(ii) Due to irregularities in the electronic configuration, there are irregularities in the enthalpies of atomization. Hence, there is irregular variation in I.E.
👉 Read Also - CBSE Class 12 Chemistry Board Exam 2025 : Most Repeated Questions from Last 10 Years; Download PDF
12. Explain the following observations:
(i) Transition elements are known to form many interstitial compounds.
(ii) The enthalpies of atomization of the transition elements are quite high.
(2012 D, 2017 Comptt. D, 2009 Delhi)
Answer ⇒
(i) The transition metals form a large number of interstitial compounds in which small atoms such as hydrogen, carbon, boron, and nitrogen occupy the empty spaces in the crystal lattices of transition metals.
(ii) Enthalpy of atomization is the amount of heat required to break the metal lattice to get free atoms. As transition metals contain a large number of unpaired electrons, they have strong interatomic attractions (metallic bonds). Hence, they have high enthalpies of atomization.
13. Chlorobenzene is extremely less reactive towards a nucleophilic substitution reaction. Give two reasons for the same. (2013 Delhi)
Answer ⇒ The reasons are:
(i) Due to resonance/diagrammatic representation, the C–Cl bond acquires a partial double bond character. As a result, the C–Cl bond in chlorobenzene is shorter and hence stronger.
Thus, cleavage of the C–Cl bond in benzene becomes difficult, which makes it less reactive towards nucleophilic substitution.
(ii) Due to repulsion between nucleophile and electron-rich arenes.
14. What are ambident nucleophiles? Explain with an example. (2014 Comptt. All India)
Answer ⇒ Ambident nucleophile: A nucleophile that can form new bonds at two or more spots in its structure, usually due to resonance contributors.
Example: S = C = N- can act as a nucleophile with either the S or N attacking.
15. Answer the following:
(i) What is known as a racemic mixture? Give an example.
(ii) Of the two bromoderivatives, C6H5CH(CH3)Br and C6H5CH(C6H5)Br, which one is more reactive in SN1 substitution reaction and why?
Answer ⇒
(i) A mixture that contains equal proportions of two enantiomers of a compound is called a racemic mixture.
Example: (±) butan-2-ol
(ii) The reactivity of SN1 reactions increases with the stability of the intermediate carbocation.
Among the two 2° bromides, the carbocation derived from C6H5CH(C6H5)Br (C6H5CH⁺C6H5) is more stable than the carbocation from C6H5CH(CH3)Br (C6H5CH⁺CH3), because it is stabilized by two phenyl groups through resonance.
16. Which halogen compound in each of the following pairs will react faster in SN2 reaction?
(i) CH3Br or CH3I
(ii) (CH3)3C—Cl or CH3—Cl
(2014 All India)
Answer ⇒
(i) CH3Br will react faster in SN2 reaction.
(ii) CH3—Cl will react faster in SN2 reaction.
👉 CBSE Class 12 Study Materials
| CBSE Class 12 Syllabus 2024-25 | CBSE Class 12 Previous Year Papers |
| NCERT Books For Class 12 Books | NCERT Class 12 Solutions |
| CBSE Class 12 Full Study Material | CBSE Class 12 Sample Paper 2024-25 |
17. Rearrange the compounds of each of the following sets in order of reactivity towards SN2 displacement:
(i) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 3-Bromo-2-methylbutane
(iii) 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane
(2011 All India)
Answer ⇒
(i) 1-Bromopentane > 2-Bromopentane > 2-Bromo-2-methylbutane
(ii) 1-Bromo-3-methylbutane > 3-Bromo-2-methylbutane > 2-Bromo-2-methylbutane
(iii) 1-Bromobutane > 1-Bromo-2-methylbutane > 1-Bromo-2,2-dimethylpropane
👉 Read Also - CBSE 12th Chemistry Exam 2025 : Important Competency Based Questions with Answers for Last Minute Revision
18. State one use each of DDT and iodoform.
Answer ⇒ Use of DDT (Dichlorodiphenyl Trichloroethane):
As a powerful insecticide, it is widely used for sugarcane and fodder crops to kill mosquitoes, lice which carry pathogens.
Use of iodoform (CHI3):
It is used as an antiseptic for dressing wounds. Its antiseptic action is due to the liberation of iodine when iodoform comes in contact with skin but not due to iodoform itself.
19. Account for the following:
Chloroform is stored in closed dark brown bottles. (2013 Delhi)
Answer ⇒ CHCl3 is stored in dark-coloured bottles to cut off light because CHCl3 is slowly oxidised by air in presence of light to form an extremely poisonous gas, carbonyl chloride, popularly known as phosgene.
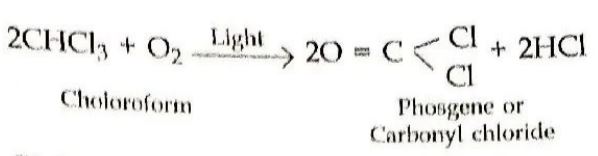
20. Write equations for Oxidation of chloroform by air and light. (2023 Series: HFG1E/5)
Answer ⇒ Oxidation of chloroform by air and light:
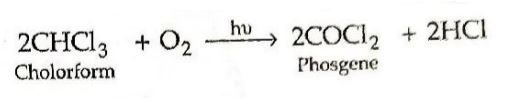
21. Non-ideal solutions exhibit either positive or negative deviations from Raoult's law. What are these deviations and why are they caused? Explain with one example for each type.
Answer ⇒
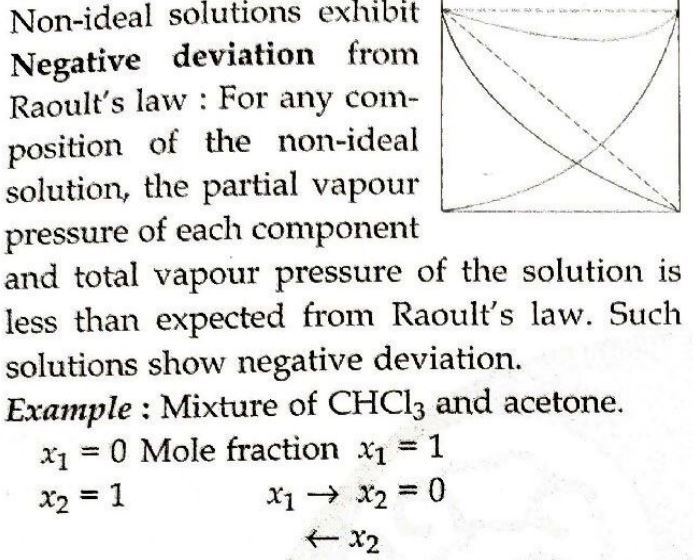
Non-ideal solutions show positive deviations from Raoult's law on mixing of two volatile components of the solution.
Example: Mixture of acetone and benzene solutions shows positive deviation.
22. A solution containing 60 g of a non-volatile solute in 250 g of water freezes at 270.67 K. Calculate molar mass of the solute. (Kf of water = 1.86 K kg mol-1) (CBSE 2024)
Answer ⇒ Given,
W2 = 60 g,
W1 = 250 g,
Kf = 1.86 K kg mol-1,
M2 = ?
Now,
ΔTf = 273 - 270.67 = 2.33 K
Using formula,
ΔTf = Kf × (W2 × 1000) / (M2 × W1)
⇒ M2 = (1.86 × 60 × 1000) / (2.33 × 250)
= 111600 / 582.5
= 191.5
∴ Molar mass of the solute, M2 = 191.5 g/mole
23. 18 g of a non-volatile solute is dissolved in 200 g of H2O freezes at 272.07 K. Calculate the molecular mass of solute (K𝑓 for water = 1.86 K kg mol-1). (CBSE 2024)
Answer ⇒ Given,
W2 = 18 g, W1 = 200 g, M2 = ?
ΔT𝑓 = T⁰𝑓 - T𝑓
⇒ ΔT𝑓 = 273.15 K - 272.07 K = 1.08 K
K𝑓 = 1.86 K kg mol-1
Using formula, ΔT𝑓 = K𝑓 m
⇒ ΔT𝑓 = K𝑓 × (W2 × 1000) / (M2 × W1)
⇒ M2 = (K𝑓 × W2 × 1000) / (ΔT𝑓 × W1)
⇒ M2 = (1.86 × 18 × 1000) / (1.08 × 200)
⇒ M2 = 33480 / 216
⇒ M2 = 155
∴ Molecular mass of solute, M2 = 155 g/mol
24. 3.9 g of benzoic acid dissolved in 49 g benzene shows a depression in freezing point of 1.62 K. Calculate the Van’t Hoff factor and predict the nature of solute (associated or dissociated).
(Given: Molar mass of benzoic acid = 122 g mol⁻¹, K𝑓 for benzene = 4.9 K kg mol-1)
Answer ⇒
ΔT𝑓 = iK𝑓 × m
⇒ ΔT𝑓 = iK𝑓 × (Wb × 1000) / (Mb × Wa)
⇒ 1.62 = i × 4.9 × (3.9 / 122) × (1000 / 49)
∴ i = (1.62 × 122 × 49) / (4.9 × 3.9 × 1000)
= 9684.36 / 19110
= 0.506
As i < 1, therefore the solute gets associated.
25. Set up Nernst equation for the standard dry cell. Using this equation show that the voltage of a dry cell has to decrease with use. (2014 Comptt. Al)
Answer ⇒ Cell reaction of a dry cell can be represented as
Zn + Hg2+ → Zn2+ + Hg
[∵ n = 2]
Nernst equation,
Ecell = E∘cell - 0.0591 / 2
log [Zn2+] / Hg2+
The voltage of a dry cell has to decrease because the concentration of electrolyte decreases in the reactions.
Download CBSE Class 12 Chemistry 2025 Important Short Type Questions with Answers PDF. Strengthen your concepts and build confidence with these essential practice questions. Download now for FREE!
| Download PDF CBSE 12th Chemistry Exam 2025 : Important Short Questions with Answers |







 Profile
Profile Signout
Signout












 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










