CONCEPT:
Identification of Functional Groups
- The reagents used in qualitative analysis help detect specific functional groups in organic compounds.
- Each reagent reacts with certain functional groups, leading to distinct observations that help identify the functional group present.
- The following steps are involved in functional group detection:
- React the compound with a specific reagent.
- Observe color changes, precipitates, or other reactions.
- Draw inferences based on known reactions of functional groups.
EXPLANATION:
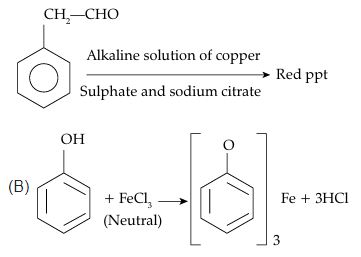
- A. Alkaline solution of copper sulphate and sodium citrate: This reagent is used to detect phenols. It gives a characteristic blue-violet coloration with phenolic compounds.
- B. Neutral FeCl3 solution: This test is also used to detect phenolic groups, which turn violet in color in the presence of FeCl3.
- C. Alkaline chloroform solution: This reagent reacts with aldehydes to give a characteristic yellow or orange precipitate.
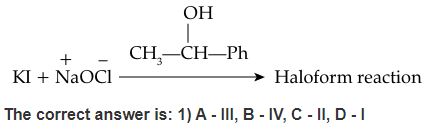
- D. Potassium iodide and sodium hypochlorite: This reagent detects alcohol groups by oxidizing them to aldehydes or ketones.
(A) Fehling’s solution is used to distinguish between aldehyde and ketone functional groups. Aldehydes oxidize to give a positive result but ketones won’t react to the test (except for a-hydroxy ketones). Fehling’s test is used as a general test for determining monosaccharides and other reducing sugars.
(C) This reaction is a chemical test for detection of primary amines, in which the amine is heated with alcoholic potassium hydroxide and chloroform. If a primary amine is present, the isocyanide is formed. The reaction is known as carbylamine reaction.
RNH2 + CHCl3 + 3KOH → RN+ ≡ C– + 3KCl + 3H2O
When a methyl ketone (even acetaldehyde) is reacted with halogen in aqueous sodium hydroxide, the ketone gets oxidised to the sodium salt of acid with one carbon less than ketone and at the same time haloform (CHX3) also gets formed.
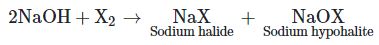
The hydroxide ion acts as a nucleophile and attacks the electrophilic carbon which is doubly bonded to oxygen. This carbon-oxygen double bond becomes a single bond making the oxygen atom anionic.


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















