
Main Concept :
First law of thermodynamicsThe first law of thermodynamics
The first law of thermodynamics is an application of the law conservation of energy, which states that energy can neither created nor destroyed, that is, the total energy of the system remains constant, though it may change from one from to another. Another statement for the first law arises from the fact that it is impossible to construct a perpetual motion machine that can produce work without spending energy on expenditure of energy.
Derivation
Suppose heat q is supplied to the system with initial internal energy U1. A part of heat energy is used by the system to do work ( - w ) while the remaining is used to change the internal energy of the system to U2. According to the first law of thermodynamics.
ΔU= U2 - U1 = q+ w
q = ΔU - ( - w )
So th energy of the system is conserved. For an infinitesimally small change dU in energy, the corresponding changes in heat and work are given by dq and dw and Eq. (2) can be written as:
dU = dq + dw
For this change in state, q and w will depend on how the change takes place. So these are not state functions. However, the change in internal energy does not depend on how a change takes place and will depend only on the initial and final states of the system. If there is no transfer of heat to or from the system (q = 0) or no work is done on or by the system (w = 0), then ΔU = 0
Question:
Express the change in internal energy of a system when
(i) No heat is absorbed by the system from the surroundings, but work (w) is done on the system. What type of wall does the system have?
(ii) No work is done on the system, but q amount of heat is taken out from the system and given to the surroundings. What type of wall does the system have?
(iii) w amount of work is done by the system and q amount of heat is supplied to the system. What type of system would it be?

Concept 1 :
Thermodynamic Processes (Isobaric, Isochoric, Isothermal and Adiabatic)
Types of Thermodynamics Process
A thermodynamic process is said to occur when the state of a system changes from one state (initial state) to another (final state).
1. Isothermal process: It is the process carried out at a constant temperature, dT = 0. For this process, the system is usually kept in contact with a constant temperature bath (thermostat) and the constant temperature is maintained by the exchange of heat with the thermostat.
2. Adiabatic process: It is the process in which heat cannot leave or enter the system, dq = 0. For this process, the system is thermally insulated from the surroundings.
3. Isobaric process: It is the process carried out at a constant pressure, dp = 0. All reactions carried out a atmospheric pressure are examples of isobaric process. However, volume change always takes place in an isobaric process.
4. Isochoric process: It is the process in which the volume of the system is kept constant (dV = 0). For example, heating of a substance in a non-expanding chamber.
5. Cyclic process: It is the process in which the initial and final states are indentical.
6. Reversible process: It is the process in which the energy change in each step of the process can be reversed in direction by making a small change in any property of the system, such as temperature, pressure, etc. Two imortant criteria for a process to be reversible are:
(a) The change must be performed at an infinitesimal slow rate.
(b) Threre must be no loss of energy due to friction and no finite temperature differences.
7. Irreversible process: It is the process in which the system or surroundings are not restored to their initial state at the end of the process. All process ocurring spontaneously in nature are irreversible. They always tend to proceed in a definite direction; and do not proceed in the opposite direction without the actions of an external force. Irreversible processes take place spontaneously and not in infinitesimal slow steps that can be reversed. Some examples of irreversible process are expansion and diffusion of gases, flow of heat from a hotter body to a colder body, etc.
Concept 2 :
EnthalpyEnthalpy
Enthalpy is the amount of heat content used or released in a system at constant pressure. Enthalpy is usually expressed as the change in enthalpy. The change in enthalpy is related to a change in internal energy (U) and a change in the volume (V), which is multiplied by the constant pressure of the system.
Enthalpy (H) is the sum of the internal energy (U) and the product of pressure and volume (PV) given by the equation:
H = U + PV
When a process occurs at constant pressure, the heat evolved (either released or absorbed) is equal to the change in enthalpy. Enthalpy is a state function which depends entirely on the state functions T, P and U. Enthalpy is usually expressed as the change in enthalpy (ΔH) for a process between initial and final states:
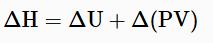
If temperature and pressure remain constant through the process and the work is limited to pressure-volume work, then the enthalpy change is given by the equation:
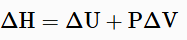
Also at constant pressure the heat flow (q) for the process is equal to the change in enthalpy defined by the equation:
ΔH = q
By looking at whether q is exothermic or endothermic we can determine a relationship between ΔH and q. If the reaction absorbs heat it is endothermic meaning the reaction consumes heat from the surroundings so q > 0 (positive). Therefore, at constant temperature and pressure, by the equation above, if q is positive then ΔH is also positive. And the same goes for if the reaction releases heat, then it is exothermic, meaning the system gives off heat to its surroundings, so q < 0 (negative). If q is negative, then ΔH will also be negative.
Enthalpy Change Accompanying a Change in State of Matter
When a liquid vaporizes the liquid must absorb heat from its surroundings to replace the energy taken by the vaporizing molecules in order for the temperature to remain constant. This heat required to vaporize the liquid is called enthalpy of vaporization (or heat of vaporization). For example, the vaporization of one mole of water the enthalpy is given as:
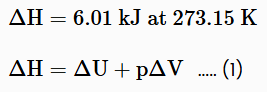
Enthalpy can also be expressed as a molar enthalpy, ΔHm, by dividing the enthalpy or change in enthalpy by the number of moles. Enthalpy is a state function. This implies that when a system changes from one state to another, the change in enthalpy is independent of the path between two states of a system.


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















