The relative rate of 3o bridgehead system towards solvolysis can be explained using, (i) strain energy & (ii) stability of carbocation intermediate.
Main Concept :
Stability of carbocation
Structure and properties
The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability (octet rule). Therefore, carbocations are often reactive, seeking to fill the octet of valence electrons as well as regain a neutral charge. One could reasonably assume a carbocation to have sp3 hybridization with an empty sp3 orbital giving positive charge. However, the reactivity of a carbocation more closely resembles sp2 hybridization with a trigonal planar molecular geometry. An example is the methyl cation, 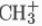 .
.
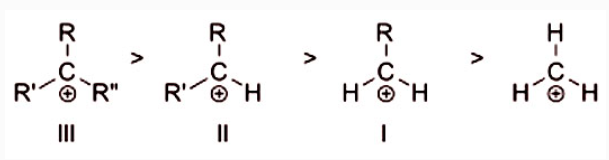
Order of stability of examples of tertiary (III), secondary (II), and primary (I) alkylcarbenium ions, as well as the methyl cation (far right). Carbocations are often the target of nucleophilic attack by nucleophiles like hydroxide (OH-) ions or halogen ions.
Carbocations typically undergo rearrangement reactions from less stable structures to equally stable or more stable ones with rate constants in excess of 109/sec. This fact complicates synthetic pathways to many compounds. For example, when 3-pentanol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearranges to a statistical mixture of the 3-pentyl and 2-pentyl. These cations react with chloride ion to produce about 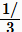 3-chloropentane and
3-chloropentane and 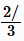 2-chloropentane.
2-chloropentane.
A carbocation may be stabilized by resonance by a carbon-carbon double bond next to the ionized carbon. Such cations as allyl cation 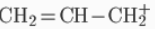 and benzyl cation
and benzyl cation 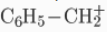 are more stable than most other carbocations. Molecules that can form allyl or benzyl carbocations are especially reactive. These carbocations where the C+ is adjacent to another carbon atom that has a double or triple bond have extra stability because of the overlap of the empty p orbital of the carbocation with the p orbitals of the π-bond. This overlap of the orbitals allows the charge to be shared between multiple atoms – delocalization of the charge - and, therefore, stabilizes the carbocation. Hyperconjugation is also a stabilizing factor for carbocations. The empty pi orbitals of the carbon atom accepts a pair of electrons from the alpha carbon which then acquires the positive charge. More alpha hydrogens increases the stability of carbocation. Stability order also follows sp3 > sp2 > sp hybridization of the carbon atom bearing positive charge
are more stable than most other carbocations. Molecules that can form allyl or benzyl carbocations are especially reactive. These carbocations where the C+ is adjacent to another carbon atom that has a double or triple bond have extra stability because of the overlap of the empty p orbital of the carbocation with the p orbitals of the π-bond. This overlap of the orbitals allows the charge to be shared between multiple atoms – delocalization of the charge - and, therefore, stabilizes the carbocation. Hyperconjugation is also a stabilizing factor for carbocations. The empty pi orbitals of the carbon atom accepts a pair of electrons from the alpha carbon which then acquires the positive charge. More alpha hydrogens increases the stability of carbocation. Stability order also follows sp3 > sp2 > sp hybridization of the carbon atom bearing positive charge
Bredt's rule - Carbocations
Bredt's rule is an empirical observation in organic chemistry that states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough. The rule is named after Julius Bredt. For example, two of the following isomers of norbornene violate Bredt's rule, which makes them too unstable to prepare:
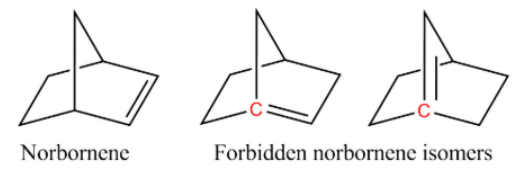
In the diagram, the bridgehead atoms involved in Bredt's rule violation are highlighted in red.
Bredt's rule is a consequence of the fact that having a double bond on a bridgehead would be equivalent to having a trans double bond on a ring, which is not possible for small rings (fewer than eight atoms) due to a combination of ring strain, and angle strain (nonplanar alkene). The p-orbitals of the bridgehead atom and adjacent atoms are orthogonal and thus are not aligned properly for the formation of ππ-bonds.
Bredt's rule can be useful for predicting which isomer is obtained from an elimination reaction in a bridged ring system. It can also be applied to reaction mechanisms that go via carbocations and, to a lesser degree, via free radicals, because these intermediates, like carbon atoms involved in a double bond, prefer to have a planar geometry with 120o angles and sp2 hybridization.
An anti-bredt molecule is one that is found to exist and be stable (within certain parameters) despite this rule. A recent (2006) example of such a molecule is 2-quinuclidonium tetrafluoroborate.


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















