When the transition element absorb violet light it emits yellow green light
Main Concept :
Examples on Colour of coordination complex ion
Description
Colours of Coordination Complexes:
Crystal Field Splitting :When ligands attach to a transition metal to form a coordination complex, electrons in the d orbital split into high energy and low energy orbitals. The difference in energy of the two levels is denoted as Δ, and it is a characteristic property of both of the metal and the ligands. This is illustrated in the "o" subscript on the Δ indicates that the complex has octahedral geometry.
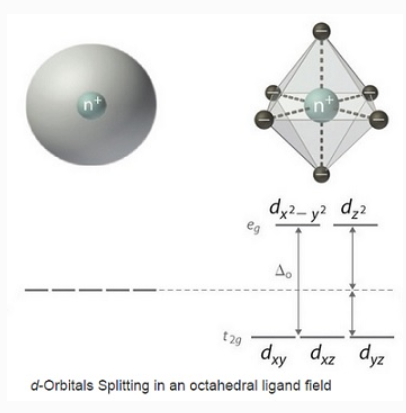
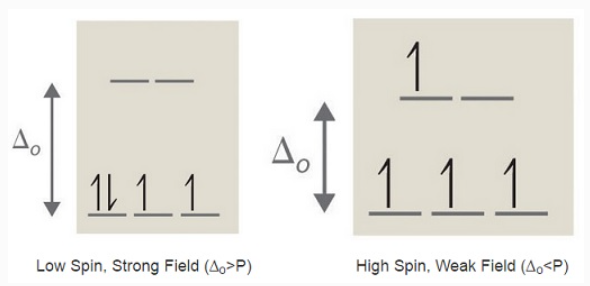
If Δ0 is large, and much energy is required to promote electrons into the high energy orbitals, the electrons will instead pair in the lower energy orbitals, resulting in a "low spin" complex; however, if Δ0 is small, and it takes little energy to occupy the higher orbitals, the electrons will do so, and remain unpaired (until there are more than five electrons), resulting in a “high spin” complex. Different ligands are associated with either high or low spin—a "strong field" ligand results in a large Δ0 and a low spin configuration, while a "weak field" ligand results in a small Δ0 and a high spin configuration. For more details, see the Crystal Field Theory (CFT) page.
A photon equal to the energy difference Δ0 can be absorbed, promoting an electron to the higher energy level. As certain wavelengths are absorbed in this process, subtractive colour mixing occurs and the coordination complex solution becomes coloured. If the ions have a noble gas configuration, and have no unpaired electrons, the solutions appear colourless; in reality, they still have a measured energy and absorb certain wavelengths of light, but these wavelengths are not in the visible portion of the EM spectrum and no colour is perceived by the eye.
In general, a larger Δ indicates that higher energy photons are absorbed, and the solution appears further to the right on the EM spectrum shown. This relationship is described in the equation 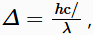 where h and c are constants, and λ is the wavelength of light absorbed
where h and c are constants, and λ is the wavelength of light absorbed
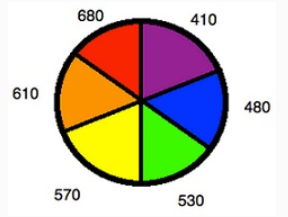
Using a colour wheel can be useful for determining what colour a solution will appear based on what wavelengths it absorbs. If a complex absorbs a particular colour, it will have the appearance of whatever colour is directly opposite it on the wheel. For example, if a complex is known to absorb photons in the orange range, it can be concluded that the solution will look blue. This concept can be used in reverse to determine Δ for a complex from the colour of its solution.
Relating the Colours of Coordination Complexes to the Spectrochemical Series
According to the Crystal Field Theory, ligands that have high spin are considered "weak field" and ligands that have low spin are considered "strong field." This relates to the colours seen in a coordination complex. High spin ligands induce the absorption of longer wavelength (lower frequency = lower energy) light than complexes with low spin ligands since their respective Δ0 values are smaller than the electron pairing energy.
The energy difference, Δ0 , determines the colour of the coordination complex. According to the spectrochemical series, the high spin ligands are considered "weak field," and absorb longer wavelengths of light (weak Δ0 ), while complexes with low spin ligands absorb light of greater frequency (high Δ0 ). The colour seen is the complementary colour of that of the wavelength absorbed. To predict which possible colours and their corresponding wavelengths are absorbed, the spectrochemical series can be used:
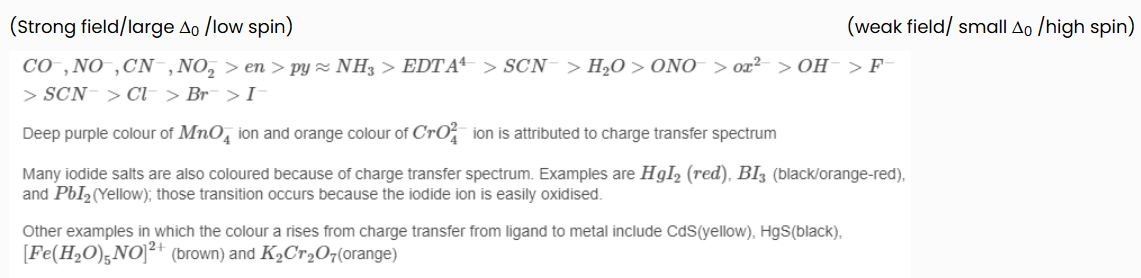


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















