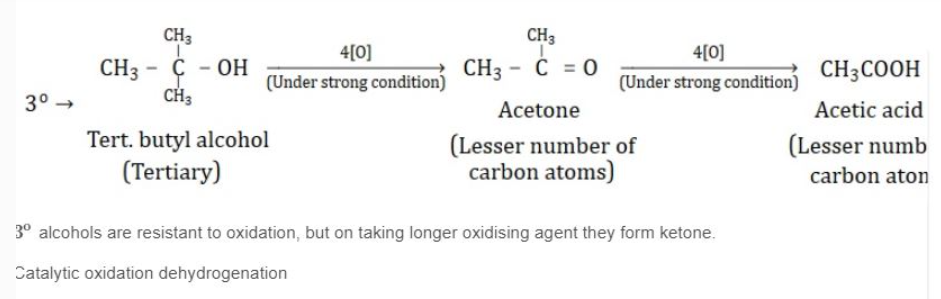
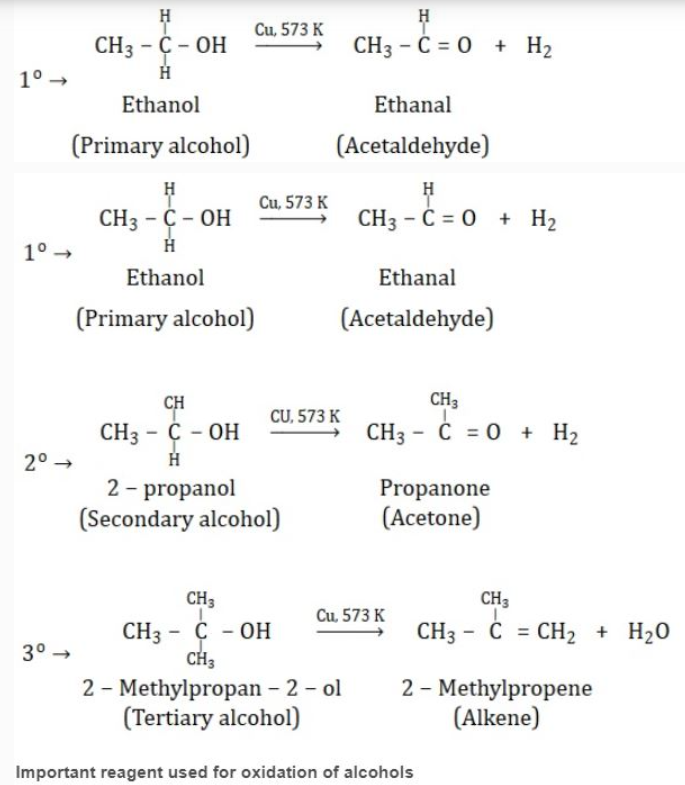
KEY CONCEPTS
Preparation of Amines
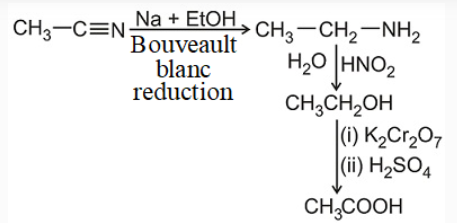
The alkylation of ammonia, Gabriel synthesis, reduction of nitriles, reduction of amides, reduction of nitrocompounds, and reductive amination of aldehydes and ketones are methods commonly used for preparing amines.
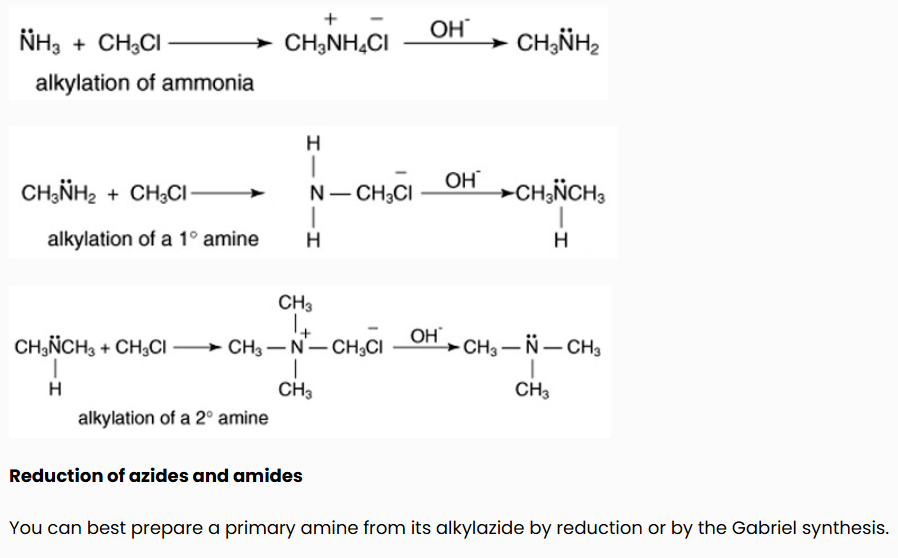
Alkylation of ammonia
The reaction of ammonia with an alkyl halide leads to the formation of a primary amine. The primary amine that is formed can also react with the alkyl halide, which leads to a disubstituted amine that can further react to form a trisubstituted amine. Therefore, the alkylation of ammonia leads to a mixture of products.
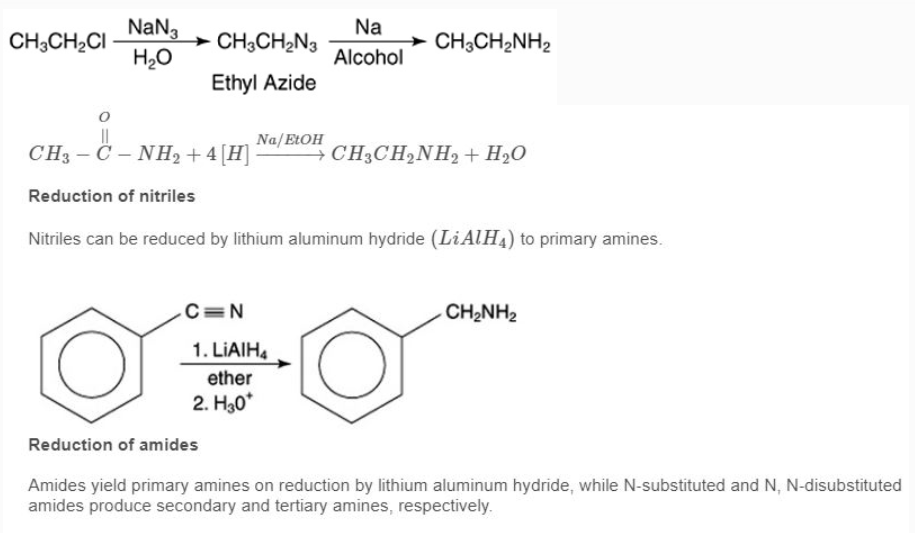
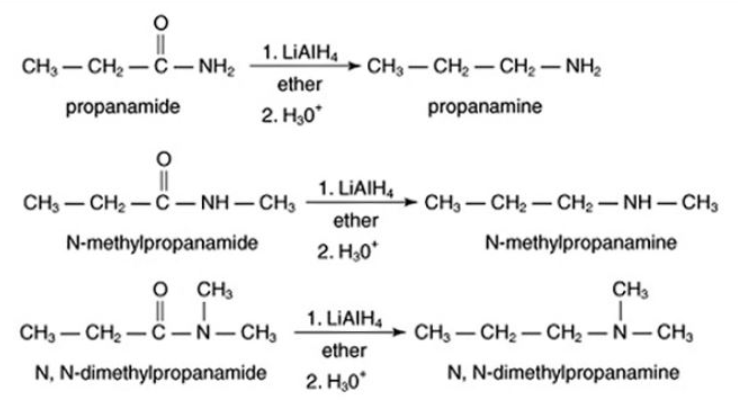
Because amides are easily prepared, their reduction is a preferred method for making all classes of amines. Reduction of nitro compounds Aromatic amines are normally prepared by reduction of the corresponding aromatic nitro compound.
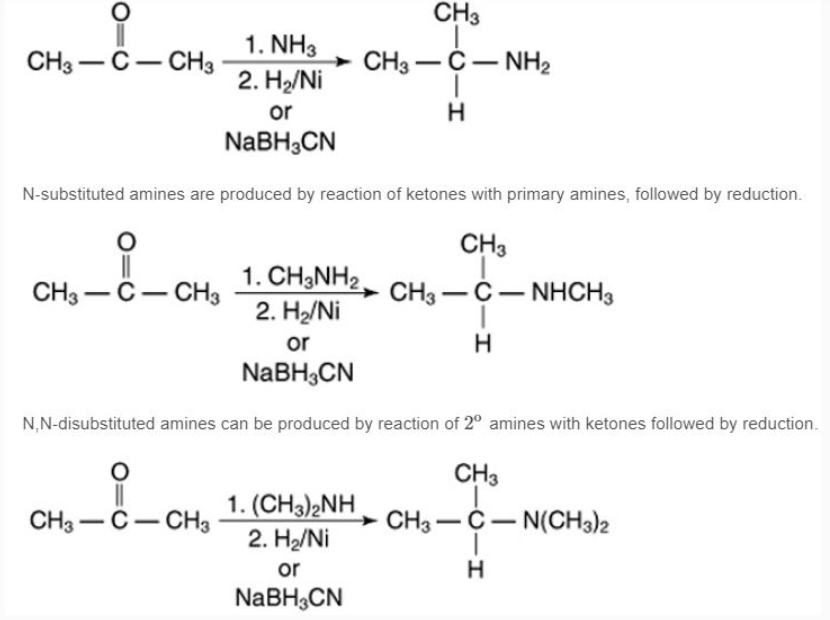
Reductive amination of aldehydes and ketonesAldehydes or ketones can be reduced by catalytic or chemical reductions in the presence of ammonia or primary or secondary amines, producing primary, secondary, or tertiary amines.The reaction of a ketone with ammonia, followed by catalytic reduction or reduction by sodium cyanoborohydride, produces a 1o amine.
Reactions of aliphatic amines with Nitrous acid
Primary amines and nitrous acid
The main observation is a burst of colourless, odourless gas. Nitrogen is given off.
Unfortunately, there is no single clear-cut equation that you can quote for this. You get lots of different organic products. For example, amongst the products you get an alcohol where the -NH2 group has been replaced by OH. If you want a single equation, you could quote (taking 1-aminopropane as an example):
CH3CH2CH2NH2 + HNO2 → CH3CH2CH2OH + H2O + N2
But the propan-1-ol will be only one product among many - including propan-2-ol, propene, 1-chloropropane, 2-chloropropane and others.
The nitrogen, however, is given off in quantities exactly as suggested by the equation. By measuring the amount of nitrogen produced, you could use this reaction to work out the amount of amine present in the solution.
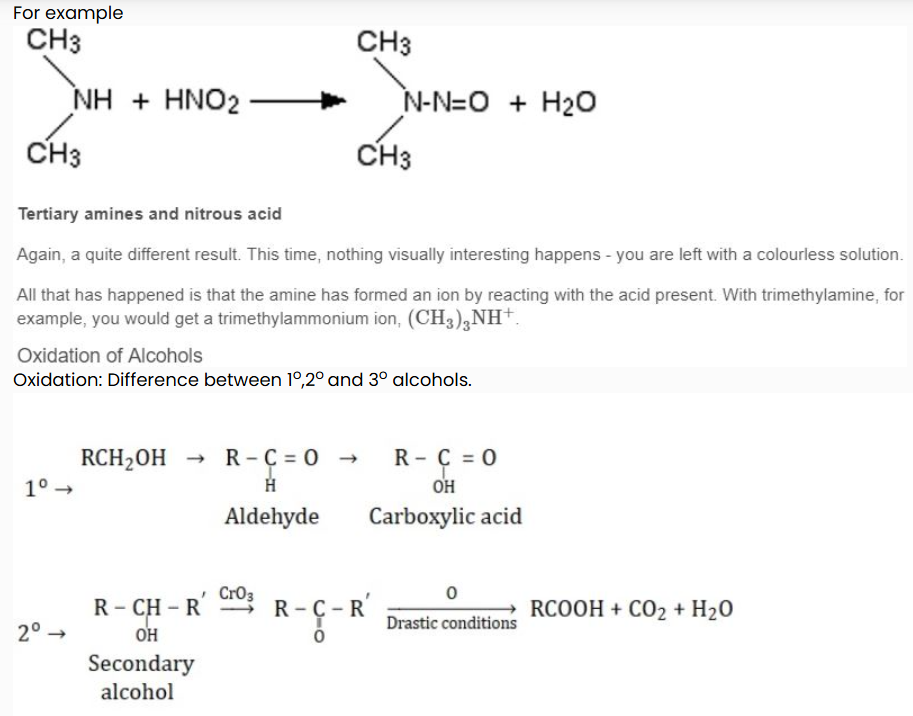
Secondary amines and nitrous acid
This time there isn't any gas produced. Instead, you get a yellow oil called a nitrosamine. These compounds are powerful carcinogens - avoid them!


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















