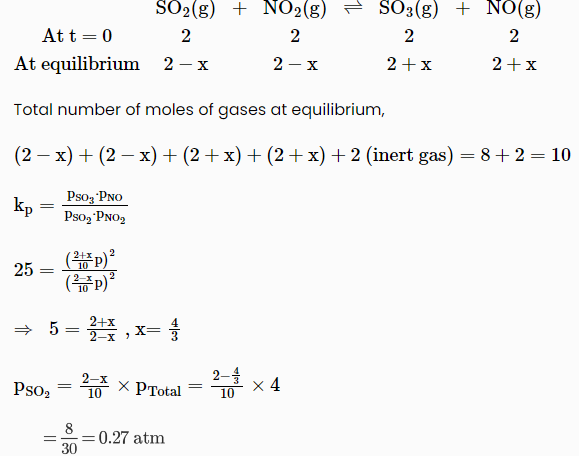
Main Concept :
Equilibrium constant expression
According to law of mass action “The rate of a chemical reaction is directly proportional to the product of the molar concentrations of the reactants at a constant temperature at any given time.”
Consider a simple reversible reaction
b (At a certain temperature)
According to law of mass action
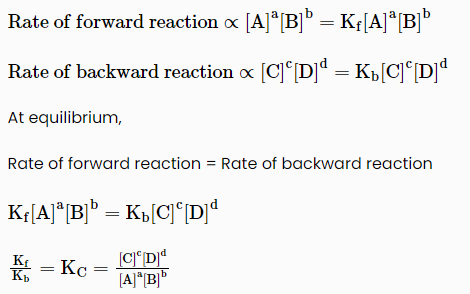
Where, KC is called equilibrium constant.
The important characteristics of equilibrium constant are discussed below:
1. The equilibrium constant has a definite value for every reaction at a particular temperature.
2. The value of equilibrium constant is independent of the original concentration of reactants.
3. The value of equilibrium constant tells the extent to which a reaction proceeds in the forward or reverse direction. If the value of K is larger, then the equilibrium concentration of the components on the right hand side of the reaction will be greater than the components on the left hand side of the reaction. Hence the reaction proceeds to a greater extent and vice versa.
4. The equilibrium constant is independent of the presence of catalyst. This is because the catalyst affects the rate of forward reaction and backward reactions equally.
5. For a reversible reaction, the equilibrium constant for the forward reaction is inverse of the equilibrium constant for the backward reaction i.e.
K forward reaction> = 1 / Kbackward reaction
6. Equilibrium constant is dependent on the temperature which is given as:
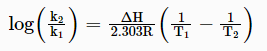
Where K1 and K2 are the equilibrium constants at temperature T1 and T2 respectively and ∆H is the enthalpy change for the reaction. It is assumed that ∆H is independent of the temperature.
Solved Examples
Q1) Consider the following reaction:
2 NO2 (g) ⇌ N2 (g) +2 O2 (g)
The equilibrium constant for the reaction at 298 K is:
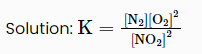


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















