For adiabatic expansion of ideal gas the relationship between b & b is b constant

Main Concept :
Thermodynamic Processes (Isobaric, Isochoric, Isothermal and Adiabatic)Types of Thermodynamics Process
A thermodynamic process is said to occur when the state of a system changes from one state (initial state) to another (final state).
1. Isothermal process: It is the process carried out at a constant temperature, dT = 0. For this process, the system is usually kept in contact with a constant temperature bath (thermostat) and the constant temperature is maintained by the exchange of heat with the thermostat.
2. Adiabatic process: It is the process in which heat cannot leave or enter the system, dq = 0. For this process, the system is thermally insulated from the surroundings.
3. Isobaric process: It is the process carried out at a constant pressure, dp = 0. All reactions carried out a atmospheric pressure are examples of isobaric process. However, volume change always takes place in an isobaric process.
4. Isochoric process: It is the process in which the volume of the system is kept constant (dV = 0). For example, heating of a substance in a non-expanding chamber.
5. Cyclic process: It is the process in which the initial and final states are indentical.
6. Reversible process: It is the process in which the energy change in each step of the process can be reversed in direction by making a small change in any property of the system, such as temperature, pressure, etc. Two imortant criteria for a process to be reversible are:
(a) The change must be performed at an infinitesimal slow rate.
(b) Threre must be no loss of energy due to friction and no finite temperature differences.
7. Irreversible process: It is the process in which the system or surroundings are not restored to their initial state at the end of the process. All process ocurring spontaneously in nature are irreversible. They always tend to proceed in a definite direction; and do not proceed in the opposite direction without the actions of an external force. Irreversible processes take place spontaneously and not in infinitesimal slow steps that can be reversed. Some examples of irreversible process are expansion and diffusion of gases, flow of heat from a hotter body to a colder body, etc.
Other Concepts :
Concept 1 :
Adiabatic reversible expansionReversible Adiabatic Expansion (or compression) of an Ideal Gas

[More generally the heat capacity for a molecular species has a temperature dependence that can be approximated as
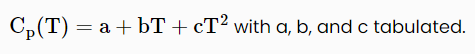


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















