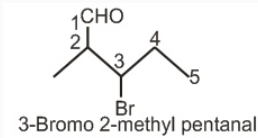
1.Find the Functional Group, here aldehyde is fuctional group.
2.Name prefix according to the priority of other groups.
Main Concept :
IUPAC names
| 1 |
Meth |
| 2 |
Eth |
| 3 |
Prop |
| 4 |
But |
| 5 |
Pent |
| 6 |
Hex |
| 7 |
Hept |
| 8 |
Oct |
| 9 |
None |
| 10 |
Dec |
In chemistry, a number of prefixes, suffixes and infixes are used to describe the type and position of functional groups in the compound.
The steps to naming an organic compound are:
I. Identification of the parent hydrocarbon chain. This chain must obey the following rules, in order of precedence:
i. It should have the maximum number of substituents of the suffix functional group. By suffix, it is meant that the parent functional group should have a suffix, unlike halogen substituents. If more than one functional group is present, the one with highest precedence should be used.
ii. It should have the maximum number of multiple bonds.
iii. It should have the maximum number of single bonds.
iv. It should have the maximum length.
II. Identification of the parent functional group, if any, with the highest order of precedence.
III. Identification of the side-chains. Side chains are the carbon chains that are not in the parent chain, but are branched off from it.
IV. Identification of the remaining functional groups, if any, and naming them by the their ion names (such as hydroxy for -OH, oxy for =O, oxyalkane for O-R, etc.).
V. Different side-chains and functional groups will be grouped together in alphabetical order. (The prefixes di-, tri-, etc. are not taken into consideration for grouping alphabetically. For example, ethyl comes before dihydroxy or dimethyl, as the "e" in "ethyl" precedes the "h" in "dihydroxy" and the "m" in "dimethyl" alphabetically. The "di" is not considered in either case). When both side chains and secondary functional groups are present, they should be written mixed together in one group rather than in two separate groups.
VI. Identification of double/triple bonds.
VII. Numbering of the chain. This is done by first numbering the chain in both directions (left to right and right to left), and then choosing the numbering which follows these rules, in order of precedence:
i. Has the lowest-numbered locant (or locants) for the suffix functional group. Locants are the numbers on the carbons to which the substituent is directly attached.
ii. Has the lowest-numbered locants for multiple bonds (The locant of a multiple bond is the number of the adjacent carbon with a lower number).
iii. Has the lowest-numbered locants for double bonds.iv. Has the lowest-numbered locants for prefixes.
VIII. Numbering of the various substituents and bonds with their locants. If there is more than one of the same type of substituent/double bond, a prefix is added showing how many there are.The numbers for that type of side chain will be grouped in ascending order and written before the name of the side-chain. If there are two side-chains with the same alpha carbon, the number will be written twice. Example: 2,2,3-trimethyl- . If there are both double bonds and triple bonds, "en" (double bond) is written before "yne" (triple bond). When the main functional group is a terminal functional group (A group which can only exist at the end of a chain, like formyl and carboxyl groups), there is no need to number it.
I. Arrangement in this form: Group of side chains and secondary functional groups with numbers made in step 3 + prefix of parent hydrocarbon chain (eth, meth) + double/triple bonds with numbers (or "ane") + primary functional group suffix with numbers.
II. Wherever it says "with numbers", it is understood that between the word and the numbers, the prefix(di-, tri-) is used.
III. Adding of punctuation:
i. Commas are put between numbers (2 5 5 becomes 2,5,5)
ii. Hyphens are put between a number and a letter (2 5 5 trimethylheptane becomes 2,5,5-trimethylheptane)
iii. Successive words are merged into one word (trimethyl heptane becomes trimethylheptane)
iv. Note: IUPAC uses one-word names throughout. This is why all parts are connected.
The finalized name should look like this:#,#-di-#--#--#,#,#-tri-#,#-di-#--#-Note: # is used for a number. The group secondary functional groups and side chains may not look the same as shown here, as the side chains and secondary functional groups are arranged alphabetically. The di- and tri- have been used just to show their usage. (di- after #,#, tri- after #,#,#, etc.)
Example: Here is a sample molecule with the parent carbons numbered:
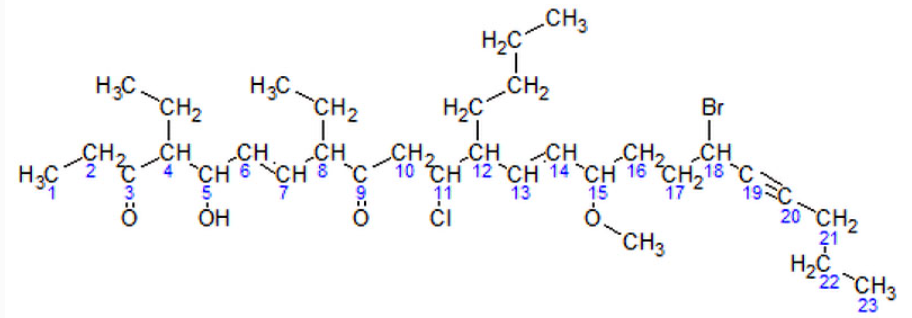
For simplicity, here is an image of the same molecule, where the hydrogens in the parent chain are removed and the carbons are shown by their numbers:
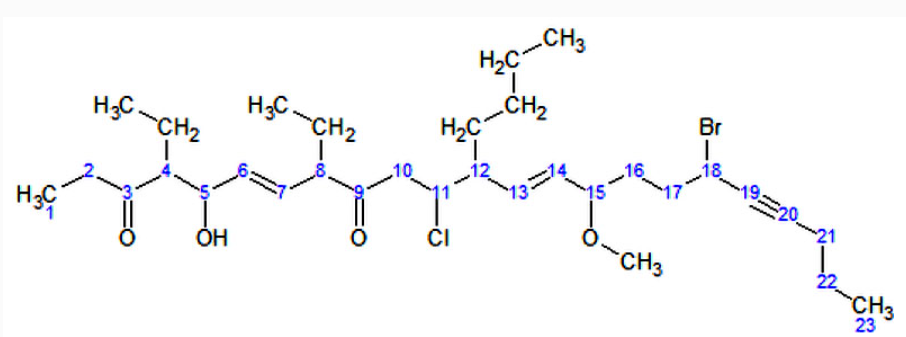
Now, following the above steps:I. The parent hydrocarbon chain has 23 carbons. It is called tricosa-.II. The functional groups with the highest precedence are the two ketone groups.i. The groups are on carbon atoms 3 and 9. As there are two, we write 3,9-dione.ii. The numbering of the molecule is based on the ketone groups. When numbering from left to right, the ketone groups are numbered 3 and 9. When numbering from right to left, the ketone groups are numbered 15 and 21. 3 is less than 15, therefore the ketones are numbered 3 and 9. The smaller number is always used, not the sum of the constituents numbers.III. The side chains are: an ethyl- at carbon 4, an ethyl- at carbon 8, and a butyl- at carbon 12. IV. Note: The -O-CH3 at carbon atom 15 is not a side chain, but it is a methoxy functional groupi. There are two ethyl- groups. They are combined to create, 4,8-diethyl.ii. The side chains are grouped like this: 12-butyl-4,8-diethyl. (But this is not the final grouping, as functional groups may be added in between.)V. The secondary functional groups are: a hydroxy- at carbon 5, a chloro- at carbon 11, a methoxy- at carbon 15, and a bromo- at carbon 18. Grouped with the side chains, this gives 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxyVI. There are two double bonds: one between carbons 6 and 7, and one between carbons 13 and 14, They would be called "6,13-diene", but the presence of alkynes switches it to 6,13-dien. There is one triple bond between carbon atoms 19 and 20. It will be called 19-yneVII. The arrangement (with punctuation) is: 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricosa-6,13-dien-19-yne-3,9-dioneVIII. Finally, due to Cis-trans isomerism, we have to specify the relative orientation of functional groups around each double bond. For this example, we have (6E,13E) The final name is (6E,13E)-18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricosa-6,13-dien-19-yne-3,9-dione.
Other Concepts :
Concept 1 :
IUPAC Rules for polyfunctional compoundsRules for IUPAC names of poly functional organic compounds
Organic elements which have two or more functional groups are called poly functional compounds. Their IUPAC names are obtained as follows,
(i) Principal functional group: If the organic compound contains two or more functional groups, one of the functional sets is selected as the principal functional group while all the remaining functional groups (also called the secondary functional groups) are treated as substituents. The following order of preference is used while selecting the principal functional group.
Sulphonic acids > carboxylic acids > anhydrides > esters > acid chlorides > acid amides > nitriles > aldehydes > ketones > thiols > alcohols >alkenes > alkynes.
All the remaining functional sets such as halo (fluoro, chloro, bromo, iodo), nitroso (- NO) -nitro (-NO2), amino (- NH2) and alkoxy (-OR) are treated as substituents.
(ii) Selecting the principal chain: Select the longest continuous chain of carbon atoms containing the principal functional group and maximum number of secondary functional groups and multiple bonds, if any.
(iii) Numbering the principal chain: Number the principal chain in such a way that the principal functional group gets the lowest possible number followed by double bond and triple bond and the substituents, i.e.
Principal functional set > double bond > triple bond > substituents
(iv) Alphabetical order: Identify the prefixes and the positional numbers (also called locants) for the secondary functional groups and other substituents and place them in alphabetical order before the word root.
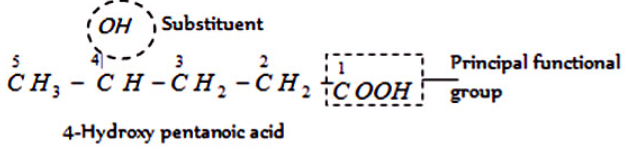
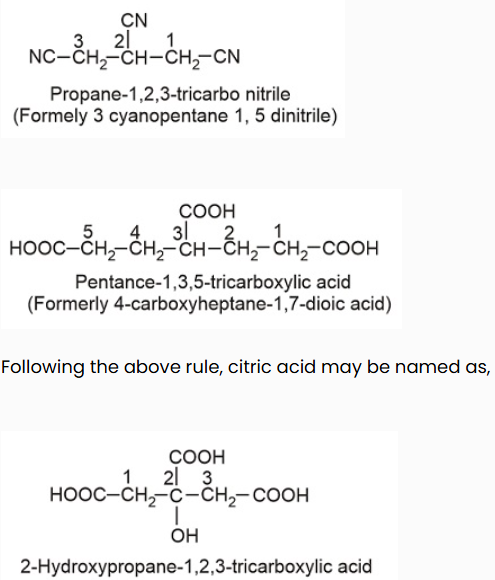
IV. Polyfunctional compounds containing more than two like functional groups : According to latest convention (1993 recommendations for IUPAC nomenclature), if an unbranched carbon reaction chain is directly linked to more than two like functional sets, the organic element is called as a derivative of the parent alkane which does not include carbon atoms of the functional sets. As given,


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















