As a given pole (N or S) of suspended magnet goes into the coil and comes out of its, current is induced in the coil in two opposite directions. Therefore, galvanometer deflection goes to left and right both. As amplitude of oscillation of magnet goes on decreasing, so does the amplitude of deflection.
KEY CONCEPTS
Lenz's law
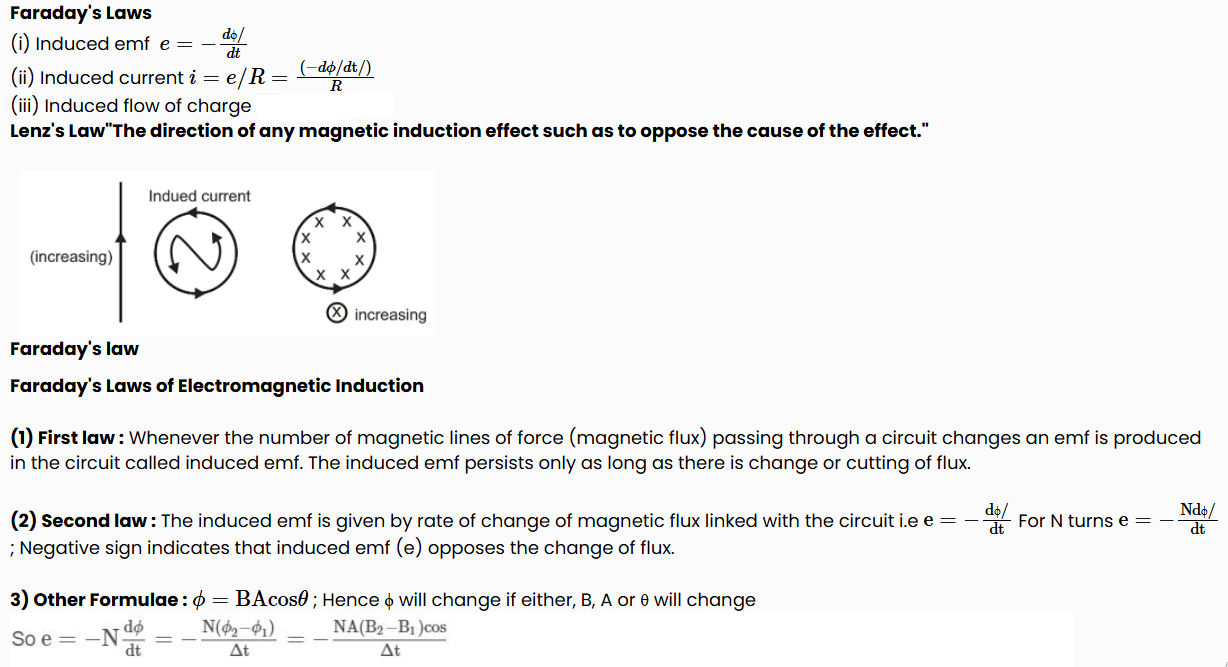
This law gives the direction of induced emf/induced current. According to this law, the direction of induced emf or current in a circuit is such as to oppose the cause that produces it. This law is based upon law of conservation of energy.
(1) When N-pole of a bar magnet moves towards the coil, the flux associated with loop increases and an emf is induced in it. Since the circuit of loop is closed, induced current also flows in it.
(2) Cause of this induced current, is approach of north pole and therefore to oppose the cause, i.e., to repel the approaching north pole, the induced current in loop is in such a direction so that the front face of loop behaves as north pole. Therefore induced current as seen by observer O is in anticlockwise direction. (figure)

Ohm's Law
If the physical conditions of the conductor (length, temperature, mechanical strain etc.) remains same, then the current flowing through the conductor is directly proportional to the potential difference across it's two ends.
I ∝ V
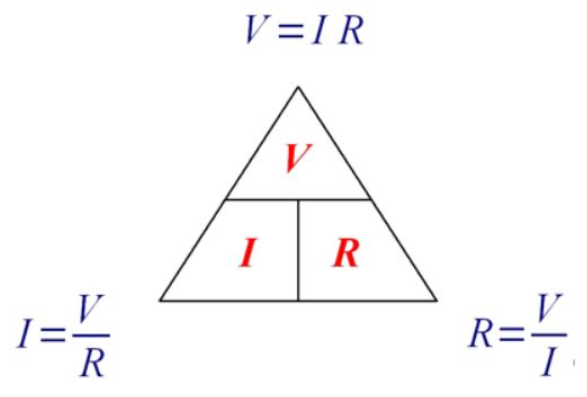
V = IR ; where R is a proportionality constant, known as electric resistance.
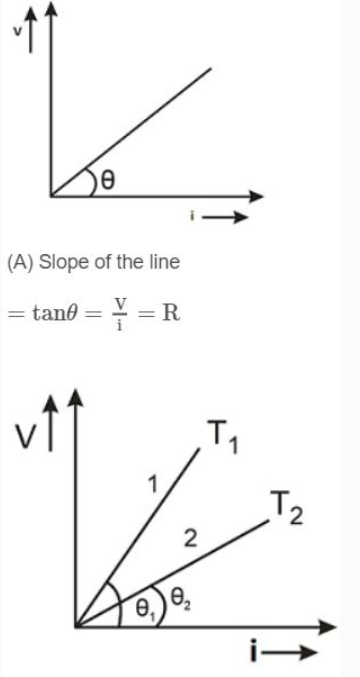
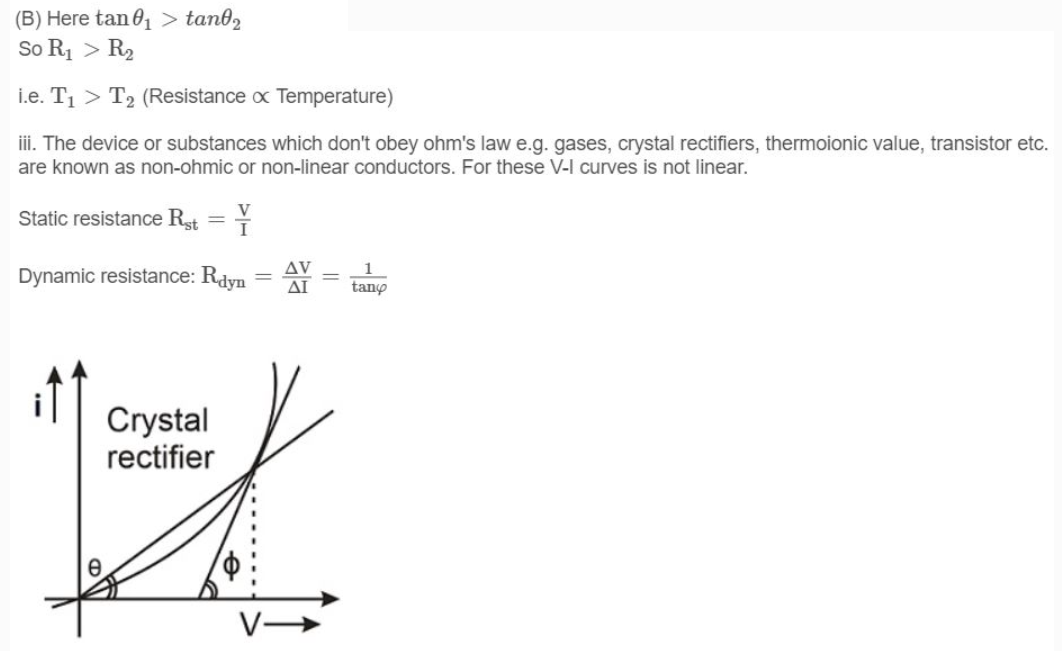
i. Ohm's law is not a universal law, the substances, which obey ohm's law are known as ohmic substance.
ii. Graph between V and i for a metallic conductor is a straight line as shown. At different temperatures V-i curves are different
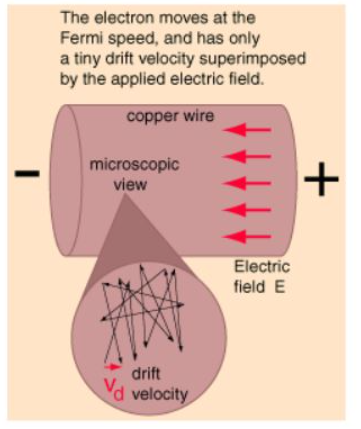
Microscopic View of Ohm's Law:
When electric current in a material is proportional to the voltage across it, the material is said to be "ohmic", or to obey Ohm's law. A microscopic view suggests that this proportionality comes from the fact that an applied electric field superimposes a small drift velocity on the free electrons in a metal. For ordinary currents, this drift velocity is on the order of millimeters per second in contrast to the speeds of the electrons themselves which are on the order of a million meters per second. Even the electron speeds are themselves small compared to the speed of transmission of an electrical signal down a wire, which is on the order of the speed of light, 300 million meters per second.

Faraday's laws of electrolysis
Faraday’s law electrolysis
Michael Faraday (1834) stated two laws on the basis of his studies on electrolysis:
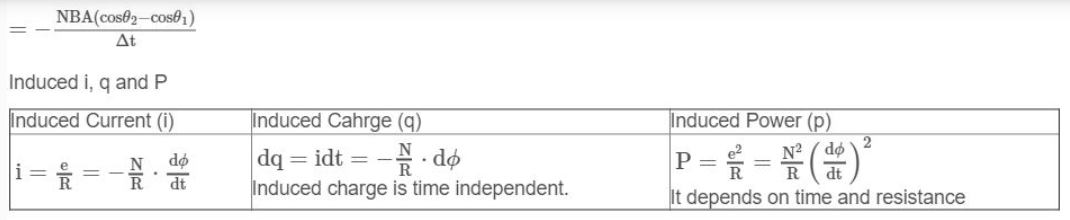
i. Faraday’s first law
According to this law, The amount of substance liberated at an electrode is directly proportional to the quantity of electricity passed.
Or M or W∝Q
Where W or M = amount of substance liberated in grams.
Q = quantity of electricity passed in coulomb.
Since Q = I.t
Where I = Current in ampere
And t = time in seconds
Hence W∝I.t or W=Z It=ZQ
Where Z = proportionality constant, called electrochemical equivalent.
If I = 1 ampere and t = 1 second then Z = W Therefore electrochemical equivalent may be defined as, “The mass of substance (in grams) liberated at the electrode on passing current of 1 ampere for 1 second or on passing 1 coulomb of electricity is called electrochemical equivalent of the substance”.



 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















