As the CBSE Class 12 board exams get closer, it’s important for students to understand the new exam pattern. Starting in the 2024-25 school year, CBSE will include 50% more competency-based questions. These questions will be both multiple choice and written, focusing on how to use what students have learned in real-life situations.
Get ready for your CBSE Class 12 Chemistry exams with our easy guide on Chapter : The Solid State. This article explores Chapter : The Solid State. It highlights key competency-based questions and provides answers to help students succeed.
Understanding Competency-Based Questions in Chapter: The Solid State
Competency-based questions are designed to see how well students can apply their knowledge in everyday life. They can come in different forms, such as case studies, true-false questions, gap-filling tasks, and long or short answer questions.
These questions are different from regular memorization. They encourage students to think critically and solve problems, helping them understand the concepts in Chapter : The Solid State better.
CBSE Class 12 Chemistry Chapter : The Solid State Important Competency-Based Questions
Q.1 The below graph shows the variation of the magnetic property of magnetite (Fe3O4) with respect to temperature.
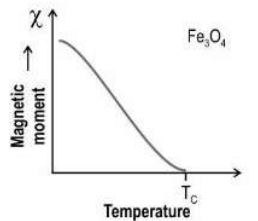
Based on this graph, which of the following represents the alignment of the magnetic moment of Fe3O4 at T >Tc?
Answer.
Q.2 Given below are two statements labeled as Assertion (A) and Reason (R).
Assertion (A): Frenkel defect is shown by compounds having a low r+/r- ratio and low dielectric constant.
Reason (R): Frenkel defect maintains the neutrality of a crystal.
Select the most appropriate answer from the options given below:
A. Both A and R are true and R is the correct explanation of A.
B. Both A and R are true but R is not the correct explanation of A.
C. A is true but R is false.
D. A is false but R is true.
Answer. D. A is false but R is true
| Download PDF | |
| CBSE Class 12 Chemistry Chapter 1 The Solid State: Important Competency-Based Questions 2024-25 | Click Here |
Q.3 Which combination of the characteristics of element X, a metal, and Y, a non-metal, is most likely to lead to ionic bonding?
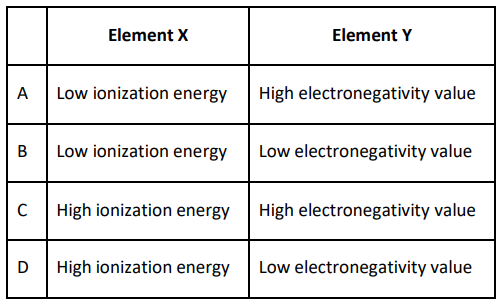
Answer. A. A
Q.4 Which of the following combinations is INCORRECT?
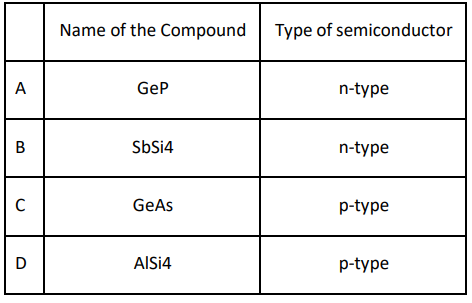
Answer. C. C
Q.5 Which of the following statements is/are true?
(i) A non-stoichiometric compound Fe0.94O is formed when 18% of Fe2+ ions are replaced by Fe3+ ions.
(ii) The conductivity of both intrinsic and extrinsic semiconductors is directly proportional to temperature.
(iii) The BCC structure is the densest crystal structure.
A. i and iii
B. ii and iii
C. only i
D. i and ii
Answer. C. only i
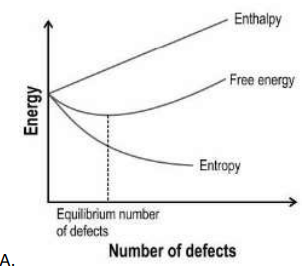
Q.6 Which of the following graphs correctly represents the enthalpy, free energy, and entropy during the formation of Schottky defects in solids?
(Hint: The overall change in free energy is given by ΔG = ΔH - TΔS)
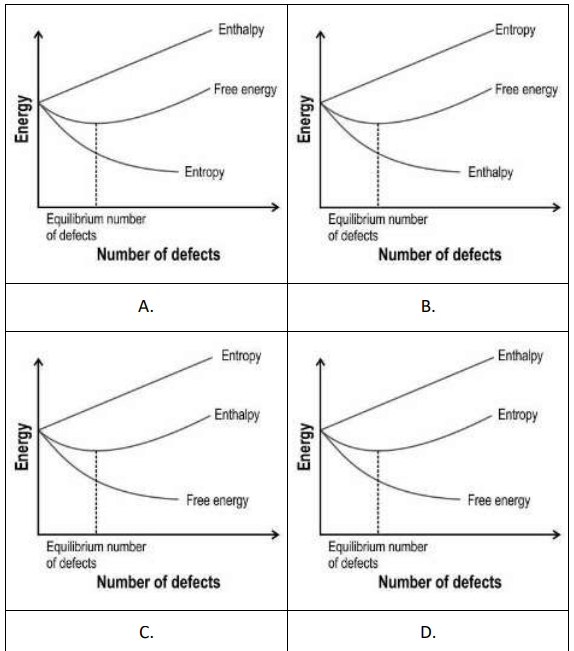
Answer.
Q.7 A compound is formed by two ions A and B in a cubic unit cell. The radius of A+ is smaller than that of B-. (as shown below)
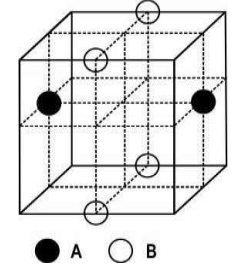
Which of the following statement is/are correct?
(i) The radius ratio, r+/r- is 0.414.
(ii) The cations and anions have different coordination geometry.
(iii) The ratio of A-B bond length to unit cell edge length is 0.866.
(iv) The formula of the compound is AB.
A. i and iii
B. iii and iv
C. ii and iv
D. All of them
Answer. B. iii and iv
Q.8 Read the statements below and answer the question based on them:
- Graphite conducts electricity and is used as a lubricant.
- Diamond is hard and does not conduct electricity.
Explain these statements on the basis of the structure and bonding present in these two solids.
Answer. Graphite:
- Each carbon atom is covalently bonded to three other carbon atoms forming flat, hexagonal rings which are arranged in layers [0.5]
- C has sp2 hybridization. Due to this the fourth valence electron is delocalized and is free to move. Free moving electrons make it a good conductor. [0.5]
- Graphite is used as a lubricant because the layers of graphite are held together by weak intermolecular/ ‘Van der Waals’ forces and hence these layers can slide over each other [1 mark] Diamond:
- Each carbon atom is covalently bonded to 4 other carbon atoms, forming a tetrahedral structure around C. C has sp3 hybridization [1 mark]
- The strong covalent bonds and tetrahedral structure and absence of delocalized electrons make diamond hard and an electrical insulator. [1 mark]
Q.9 KCl crystallizes in the same type of lattice as NaCl. If rNa+/rCl- = 0.5 and rNa+/rK+ = 0.7.
What is the ratio of the side of the NaCl unit cell to that of the KCl unit cell?
Answer. Calculating ratio of the side of NaCl to that of KCl:
- NaCl crystallizes in fcc unit such that rNa++ rCl- = a/2 (assuming a is side length of an unit cell for NaCl)[0.5 marks]
- Given that rNa+ / rCl- = 0.5
and rNa+/rK+ = 0.7, thus
(rNa+ + rCl-)/rCl- = 1.5 and
rK+/rCl- = 0.5/0.7 [0.5 marks]
- Using the above equations:
(rK+ + rCl-)/(rNa+ + rCl-) = (1.2/0.7) x (1/1.5)
∴ aNaCl : aKCl = 1:1.143 [1 mark]
Q.10 Explain why crystalline solids are generally MORE DEFECTIVE at high temperatures. (Hint: Use the Gibbs energy equation)
Answer. - As per the Gibbs-Helmholtz equation:
ΔG = ΔH − TΔS;
To create defects, the enthalpy of formation must be provided. [1 mark]
- A large positive increase in entropy will be associated with the defect.
- At high temperatures, it is more likely that the term TΔS > ΔH, and thus ΔG < 0 and defects may form at thermodynamic equilibrium. [1 mark]
Q.11 In an FCC lattice, with the help of a diagram, show that the minimum distance between an octahedral void and a tetrahedral void is (√3/4)a.
(Note: a is the side length of the unit cell)
Answer. Calculating the shortest distance between an octahedral void and a tetrahedral void in FCC solid: - Draw the diagram of one-unit cellshowing the position of the octahedral void, and tetrahedral void as below: [1 mark]
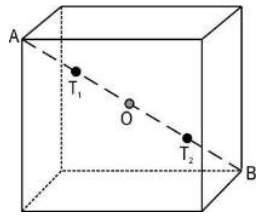
- In the above figure, AB isthe diagonal of the cube, T1 and T2 are tetrahedral voids, and O is the octahedral void at the centre of the cube. [0.5 mark]
- In FCC, tetrahedral voids are located at 1/4th distance from the corner along the diagonal. So, AT1 = AB/4 - Since AB = √3a; AT1 = √3a/4 [1 mark]
- Since the octahedral void is at the centre of the cube/diagonal. So, AO = AB/2
∴ AO = √3a/2 [0.5 mark]
- Now the distance between an octahedral void and a tetrahedral void = AO - AT1
= √3a/2 - √3a/4 = √3a/4 [ 1 mark]
Q.12 The diagram below shows the location of octahedral void per unit cell at the body center and at the center of one edge of the unit.
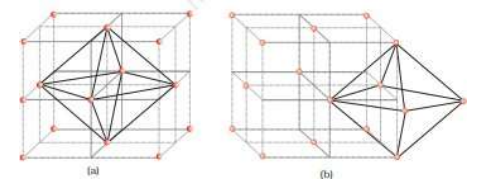
Ifthe distance between the two nearest octahedral voidsis'√2p' cm, where p is any positive number.
(i) What is the minimum distance between the two tetrahedral voids in the same unit cell?
(ii) What is the maximum distance between the two tetrahedral voids in the same unit cell?
Answer. (i) minimum distance between the two tetrahedral voids
- minimum distance between the two octahedral voids = a/√2; where a is the side of the unit cell.
- a/√2 = √2p ; p is a positive number
∴ a = 2p [1 Mark]
So, the minimum distance between the two tetrahedral voids = a/2 = p [1 mark]
(ii) the maximum distance between the two tetrahedral voids
- the maximum distance between the two tetrahedral voids = √3a/2 ; where a is the side length of unit cell
- so maximum distance = √3p [1 mark]
Q.13 In a crystal, there are N possible cation and anion sites. If there are nc cation vacancies and na anion vacancies in the same crystal, then what is the number of ways in which one can distribute:
(i) Cation vacancies
(ii) Anion vacancies
(iii) Total number of ways of distributing these defects
Answer. (i) No. of ways one can distribute cation vacancies:
- Probability theory shows that the no. of ways of distributing n defects over N sites
= N!/(N-n)!n!
∴ No. of ways one can distribute cation vacancies = N!/(N-nc)!nc! [0.5 mark]
(ii) Similarly, No. of ways one can distribute anion vacancies = N!/(N-na)!na! [0.5 mark]
(iii) Total number of ways of distributing these defects = N!/(N-nc)!nc! x N!/(Nna)!na! [1 mark]
Q.14 A compound is formed by two elements M and N. The element M forms fcc lattice and N occupies all the octahedral voids.
If all the atoms along the 011 plane (as shown below in grey) are missing, then derive the formula of the compound?
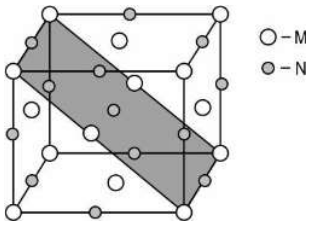
Answer. Finding the formula of the compound:
- In the new arrangement the no of atoms of M = (1/8 x 8 + 1/2 x 6 ) - ( 1/8 x 4 + 1/2 x 2) = 5/2 [1 mark]
- the no. of atoms of N = (1/4 x 12 +1) - (1/4 x 2 +1) = 5/2 [1 mark]
- So the new formula = MN [1 mark for correct answer]
Q.15 The diagram below shows a part of the structure of a crystal with some ions missing.
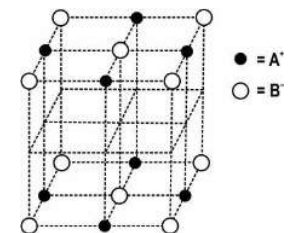
(i) Complete the diagram by placing cation A and anion B at appropriate sites.
(ii) Identify the formula of this crystal.
Answer. (i) Diagram
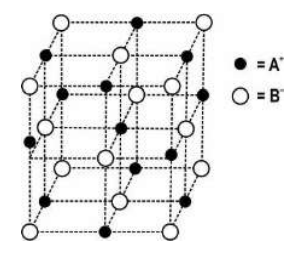
(ii) Formula:
-No. of atoms A = (1/4 x 12 + 1) = 4 [0.5 mark]
- No. of atoms B = 1/8 x 8 + 1/2 x 6 = 4 [0.5 mark]
- Formula = AB [1 mark]
-
👉 Read Also - CBSE 2025 Exam Date Sheet (Out) : CBSE Class 10, 12 Time Table Released – Exams Begin Feb 15
👉 Read Also - How CBSE’s New Exam Pattern Will Impact Class 11 and 12 Students
👉 CBSE Class 12 Study Materials
| CBSE Class 12 Syllabus 2024-25 | CBSE Class 12 Previous Year Papers |
| NCERT Books For Class 12 Books | NCERT Class 12 Solutions |
| CBSE Class 12 Full Study Material | CBSE Class 12 Sample Paper 2024-25 |







 Profile
Profile Signout
Signout








 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










