Explanation-
Wurtz reaction-
- Wurtz’s reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen.
The general form of the Wurtz reaction equation can be written as follows:
2R-X + 2Na → R-R + 2Na+ X–
- It can be observed from this equation that the two R groups are joined, yielding an alkane with a longer chain along with NaX, where X is a Halogen.
Given data and Analysis-
\(2\rm C_2H_5Br +Na\xrightarrow{Na/Dry\ ether}C_2H_5-C_2H_5 +2NaBr\)
→ It can be observed from this equation that the two R groups are joined, yielding an alkane with a longer chain along with NaBr, where Br is a Halogen.
So it is Wurtz's reaction.
 Important Points
Important Points
Difference between Wurtz and Ullmann reaction-
- The Wurtz–Fittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds as their products.
- The Ullmann reaction or Ullmann coupling is a coupling reaction between aryl halides and copper.
 Additional Information
Additional Information
Sandmeyer's reaction-
Sandmeyer reaction is a type of substitution reaction that is widely used in the production of aryl halides from aryl diazonium salts. Copper salts like chloride, bromide, or iodide ions are used as catalysts in this reaction.
Notably, the Sandmeyer reaction can be used to perform unique transformations on benzene. The transformations include hydroxylation, trifluoromethylation, cyanation, and halogenation.
Williamson's synthesis
- The general method for the synthesis of ether is Williamson ether synthesis, which involves nucleophilic displacement of a halide ion or other good leaving group by an alkoxide ion.
- The basic mechanism of the reaction is: R-O- + R'-X → R-O-R + X–
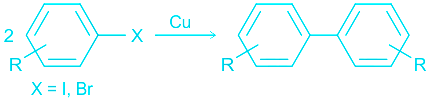
Ullmann reaction
The Ullmann reaction (also known as Ullmann coupling) is an organic named reaction that involves the coupling of two aryl halides in the presence of copper to yield a biaryl as the product.
The general format of the Ullmann reaction is illustrated below.


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















