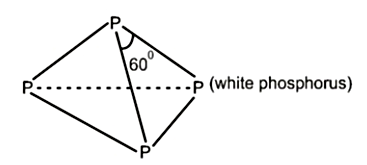
As valency of phosphorus is 5 and there are in total four phosphorus atoms. Each \(P\) atom has one lone pair of electron and each covalent bond (includes two electrons) will be formed Therefore, in total there will be 4 lone pair of electrons and six P-P single bonds in a molecule of white phosphorus.
\(P_4\) has six \(P− P\) single bonds.
Properties of Phosphorus \(\left(P_{4}-\text { White }\right)\)
White or slightly yellow, waxy, non-conductor of heat and electricity, insoluble in water but soluble in many organic solvents.
It is highly reactive owing to the large angle strain in the tetrahedral structure of \(P_{4}\).
Allotropy of elements of nitrogen family group (VA) Allotropy: All the members of group \(15\) except \(Bi\) exhibit the phenomenon of allotropy.
Nitrogen exists in two solid and one gaseous allotropic forms.
Phosphorus exists in several allotropic forms such as white, red, scarlet, violet and black form.
a. White or yellow phosphorus: White phosphorus is prepared from rock phosphate \(\mathrm{Ca}_{3}\left(\mathrm{PO}_{4}\right)_{2}, \mathrm{SiO}_{2}\) and coke which is electrically heated in a furnace.
\(2 \mathrm{Ca}_{3}\left(\mathrm{PO}_{4}\right)_{2}+6 \mathrm{SiO}_{2} \stackrel{\Delta}{\rightarrow} 6 \mathrm{CaSiO}_{3}+\mathrm{p}_{4} \mathrm{O}_{10}\)
\(\mathrm{P}_{4} \mathrm{O}_{10}+10 \mathrm{C} \stackrel{\Delta}{\rightarrow} \mathrm{P}_{4}+10 \mathrm{CO}\)
When exposed to light, it acquires a yellow colour.
b. Red phosphorus: It is obtained by heating yellow phosphorus, between \(240-250^{\circ} \mathrm{C}\) in the presence of inert gas. Yellow phosphorus can be separated from red phosphorus by reaction with \(\mathrm{NaOH}\) (aq) or \(\mathrm{KOH}\) (aq) when the former reacts and the latter remains unreacted.
Arsenic exists in three allotropic forms namely grey, yellow and black. Antimony also exists in three forms, viz, metallic, yellow and explosive.


 Get latest Exam Updates
Get latest Exam Updates 
 ×
×
















