CBSE Class 10 Science 2024-25: Chapter 1 Chemical Reactions & Equation Important Competency-Based Questions with Answers; Download Free PDF

SHARING IS CARING
If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
As the CBSE Class 10 board exams get closer, it’s important for students to understand the new exam pattern. Starting in the 2024-25 school year, CBSE will include 50% more competency-based questions. These questions will be both multiple choice and written, focusing on how to use what students have learned in real-life situations.
This article explores Chapter 1: Chemical Reactions & Equations. It highlights key competency-based questions and provides answers to help students succeed. Prepare for your CBSE Class 10 Science exams with important competency-based questions from Chapter 1: Chemical Reactions & Equations.
Understanding Competency-Based Questions in Chapter 1: Chemical Reactions & Equations
Get ready for the CBSE Class 10 Science 2024-25 exams with key competency-based questions from Chapter 1: Chemical Reactions & Equations. These questions help you apply concepts in real-life situations. Download the free PDF for detailed answers and boost your exam preparation.
CBSE Class 10 Science Chapter 1: Chemical Reactions & Equations Important Competency-Based Questions
Q1. Which of the following is an example of simple displacement?
1. the electrolysis of water
2. the burning of methane
3. the reaction of a metal with an acid
4. the reaction of two salt solutions to form a precipitate
Ans. 3. the reaction of a metal with an acid
Q2. Which of the following is a NECESSARY condition for ALL chemical reactions?
1. The reactants should be in the same state.
2. Energy should be supplied to the reactants.
3. The reactants should be at the same temperature.
4. There should be physical contact between the reactants.
Ans. 4. There should be physical contact between the reactants.
Q3. Given below is the balanced chemical equation for the thermal decomposition of lead nitrate.
2 Pb(NO3)2 ---> 2 PbO + 4 NO2 + 02
Which of the following information does the coefficients of PbO and NO, in the equation (2 and 4 respectively) tell us?
1. the ratio of the number of moles produced of the two substances
2. the ratio of the number of atoms in the two substances
3. the ratio of the mass produced of the two substances
4. the ratio of the densities of the two substances
Ans. 1. the ratio of the number of moles produced of the two substances
| Download PDF | |
| CBSE Class 10 Science Chapter 1: Important Competency-Based Questions | Click Here |
Q4. The diagram below shows the set-up in which electrolysis of water takes place.
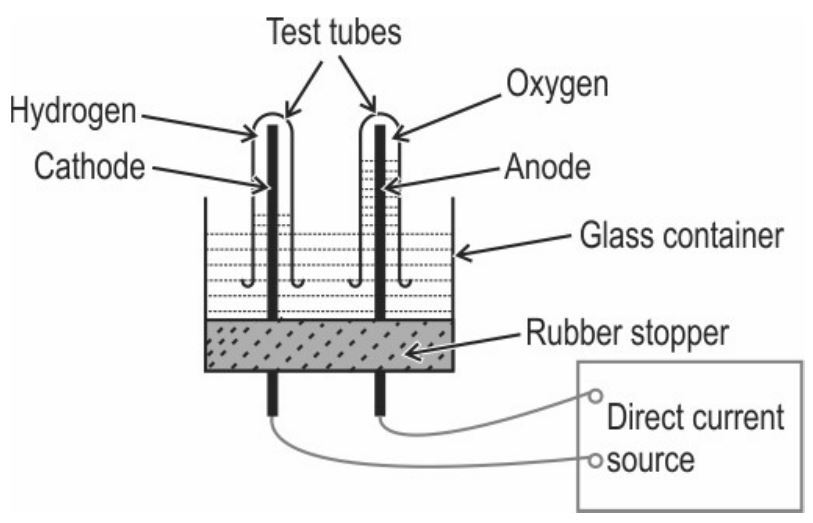
(a) What type of reaction takes place?
(b) Explain why this is an example of an endothermic reaction?
(c) The test tube containing hydrogen is removed carefully from the apparatus. A lit match stick is brought near the mouth of this test tube. The gas burns with an explosive "pop" sound.
Write a balanced chemical equation for this reaction and indicate whether energy is absorbed or released.
Ans.
(a) Decomposition / Electrolytic decomposition
(b) Energy in the form of electrical energy is absorbed during the decomposition of water.
(c) Balanced equation:
2H₂O + energy ---> 2H₂ + O₂
- 0.5 marks for correctly mentioning the reactants and products
- 0.5 marks for balancing the reaction
- 0.5 marks for showing the endothermic nature of the reaction
Q5. Eight identical, iron blocks are placed on the ground in the two arrangements X and Y as shown below. The block arrangements are kept moist by sprinkling water every few hours
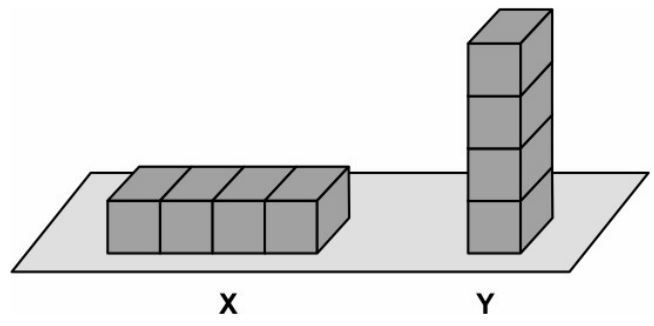
Which of the arrangements is likely to gather more rust after ten days? Justify your answer.
Ans.
- arrangement Y
- Rusting is a surface phenomenon.
- Arrangement Y has a larger surface area exposed to air.
Q6. The following chemical equation does not represent a chemical reaction that can take place.
3 Fe (s) + 4 H₂O (I) ------> Fe3 04 (s)
State what needs to be changed in the equation above for it to represent the correct reaction between Fe and H₂O.
Ans. The water should be in the form of steam, not liquid.
Q7. Trupti mixes an aqueous solution of sodium sulphate (Na₂ SO4) and an aqueous solution of copper chloride (CuCl₂).
Will this lead to a double displacement reaction? Justify your answer.
Ans. There will be no reaction.
1 mark for either of the following:
- All the ions will be in solution.
- There is no insoluble product formed on mixing the two solutions.
Q8. Dilip was comparing combination reactions with decomposition reactions.
Which class of chemical substances may be the product of a decomposition reaction but NOT a product of a combination reaction?
Ans. element
Q9. Write the balanced chemical equation of any one reaction that CANNOT be classified as combination, decomposition, simple displacement or double displacement.
Ans. 1 mark for any correct example such as:
CH4 + 2 0₂ ---> CO2 + 2 H₂O
6 CO₂ + 6 H₂O ---> C6 H12 O6 + O₂
Q10. Tina finds a paper covered with a white substance in a chemistry lab. She keeps the paper near the window of the lab and comes back to pick it up after five hours to take it home. She noticed that the white substance had turned grey.
(a) What could be the most likely substance on the paper that Tina found?
(b) The substance changed from white to grey. Write the chemical equation for this reaction.
(c) State ONE application of this property of the substance seen in daily life.
Ans. (a) silver chloride (AgCI) / silver bromide (AgBr)
Q11. Photographic film consists of a gelatin emulsion with silver halide grains layered onto a film base. The halidesthat are used are silver chloride, bromide or iodide. The photographic film is usually stored in metal containers to protect it from light.
Write the chemical equation forthe possible chemicalreaction that this method of storing photographic film is preventing.
Ans. 
Q12. Trupti mixed one teaspoon of baking soda in 500 g of cake mixture. She kept the mixture aside for 5 minutes.
Geeta mixed one teaspoon of baking powder in 500 g of the same cake mixture. She also kept the mixture aside for 5 minutes.
She then baked the two cakes together in the same oven. Whose cake is likely to rise higher? Justify your answer.
Ans. Trupti's cake will rise more.
Baking powder is a mixture of sodium bicarbonate and tartaric acid.
- Baking soda is pure sodium bicarbonate. Hence one teaspoon of baking soda contains more bicarbonate than baking powder and releases more carbon dioxide than baking powder.
Q13. While cooking in an aluminum vessel, Sudeshna burned some food till all that wasleft was a completely charred and black residue. She just left the blackened vessel heating on the stove. After an hour she found that the vessel was completely clean, with no trace of any blackness.
(a) Write a chemical equation to explain what happened to the charred, black residue that made it disappear.
(b) Name the type of reaction referred to in (a).
Ans. (a) 0.5 marks each for writing the reactants and product:
C + O2 ---> CO2
(b) 0.5 marks for any of the following:
- combustion
- oxidation
- combination
👉 Read Also- CBSE Class 10 Half-Yearly/Mid Term 2024-25 : Most Important Questions with Answers; PDF Download (All Subjects)
👉 CBSE Class 10 Study Materials
| CBSE Class 10 Syllabus 2024-25 | NCERT Solutions For Class 10 |
| CBSE Class 10 Previous Year Question Papers | CBSE Class 10 Books |
| CBSE Class 10 Full Study Material | CBSE Class 10 Sample Paper |







 Profile
Profile Signout
Signout












 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










