CBSE Class 10 Science 2024-25: Chapter 4 Carbon and its Compounds Competency-Based Questions with Answers; Download Free PDF

SHARING IS CARING
If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
As the CBSE Class 10 board exams get closer, it’s important for students to understand the new exam pattern. Starting in the 2024-25 school year, CBSE will include 50% more competency-based questions. These questions will be both multiple choice and written, focusing on how to use what students have learned in real-life situations.
This article explores Chapter 4: Carbon and its Compounds. It highlights key competency-based questions and provides answers to help students succeed. Prepare for your CBSE Class 10 Science exams with important competency-based questions from Chapter 4: Carbon and its Compounds.
Understanding Competency-Based Questions in Chapter 4: Carbon and its Compounds
Get ready for the CBSE Class 10 Science 2024-25 exams with key competency-based questions from Chapter 4: Carbon and its Compounds. These questions help you apply concepts in real-life situations. Download the free PDF for detailed answers and boost your exam preparation.
CBSE Class 10 Science Chapter 4: Carbon and its Compounds Important Competency-Based Questions
Q: 1 On undergoing complete combustion in an adequate supply of oxygen, an organic compound produces only carbon dioxide and water vapour as the products.
Based on this information, which of the following homologous series could the
compound belong to?
P) alkanes
Q) alcohols
R) aldehydes
1. only P
2. only P or Q
3. only Q or R
4. any P, Q or R
Answer. (4)
Q: 2 1 mole of ethene and 1 mole of ethyne are separately made to completely undergo addition reaction to form the respective saturated compound.
Which of the following will be DIFFERENT for the two reactions?
P) the number of moles of the saturated compound formed
Q) the number of moles of the hydrogen consumed
1. only P
2. only Q
3. both P and Q
4. neither P nor Q
Answer. (2)
Q: 3 Two statements are given - one labelled Assertion (A) and the other labelled Reason
(R). Read the statements carefully and choose the option that correctly describes statements A and R.
Assertion (A): Vegetable oils are healthier than animal fats.
Reason (R): Vegetable oils generally have long unsaturated carbon chains while animal fats have saturated carbon chains.
1. Both A and R are true and R is the correct explanation for A.
2. Both A and R are true and R is not the correct explanation for A.
3. A is true but R is false.
4. A is false but R is true.
Answer. (1)
Q: 4 Bromine water is a reddish solution of bromine (Br) in water. When shaken with an unsaturated hydrocarbon, the red colour of the bromine water disappears because the bromine is used up in an addition reaction.
Kohli has three test tubes containing hexane, hexene and hexyne respectively. Which of the three compounds can he identify using the bromine water test? Give a reason for your answer.
Answer. - hexane [0.5 marks]
- Only hexane will not decolourise the bromine water. [0.5 marks]
| Download PDF | |
| CBSE Class 10 Science Chapter 1 Chemical Reactions & Equation: Important Competency-Based Questions 2024-25 | Click Here |
| CBSE Class 10 Science Chapter 2 Acids, Bases and Salts: Important Competency-Based Questions 2024-25 | Click Here |
| CBSE Class 10 Science Chapter 3 Metal and Non-metal: Important Competency-Based Questions 2024-25 | Click Here |
| CBSE Class 10 Science Chapter 4 Carbon and its Compounds: Important Competency-Based Questions 2024-25 | Click Here |
Q: 5 A carbon compound of molecular formula C5 H10 O contains a ketone functional group.
Draw the structures of three isomers of this compound having a ketone group.
Answer.
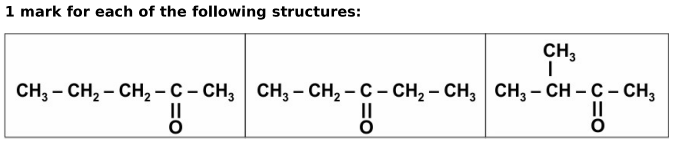
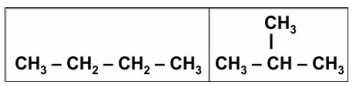
Q: 6 Compounds with identical molecular formula but different structures are called structural isomers.
(a) In the case of saturated hydrocarbons, what is the MINIMUM number of carbon atoms needed in a molecule for it to have a structural isomer?
(b) Draw the structural isomers of the saturated hydrocarbon having the minimum number of carbon atoms mentioned in (a).
Answer. (a) four
(b) 1 mark each for the following isomers:
Q: 7 Organic compounds belonging to different homologous series can be isomers. For example, propanal and propanone are isomers.
Can an alkane and an alcohol be isomers? Why or why not?
Answer. 1 mark for each of the following points:
- No, they cannot be isomers.
- Alkanes have only carbon and hydrogen atoms, while alcohols have oxygen atoms too.
Q.8 Combustion analysis of compound X revealed the ratio of its elements in a molecule to be carbon:hydrogen:oxygen::3:6:2. The compound undergoes esterification to yield an ester and water.
(a) Identify the compound X. Write its chemical formula.
(b) Write the balanced equation for the combustion of compound X.
Answer. (a) Propionic acid / Propanoic acid [0.5 marks]
C2H5COOH [0.5 marks]
(c) 2C2H5COOH + 7O2 --> 6CO2 + 6H2O + energy
Q.9 Bonding of oxygen with carbon and with hydrogen are highly exothermic processes. Compare the amount of energy released on combustion of methane and propane.
(a) Which compound will yield more energy per mole on combustion? Justify your answer.
(b) Write the balanced chemical equationsfor complete combustion of propane and methane.
Answer. (a) Energy released permole will be more in propane since it has more number of carbon atoms.
(b) 1 mark for each correct equation:
CH4 + 2O2 --> CO2 + 2H2O
C3H8 + 5O2 --> 3CO2 + 4H2O
Q.10 One mole of an alkane P is burned in an excess of oxygen to yield 6 moles of carbon dioxide and 14 moles of water.
(a) Write the chemical formula and chemical name of the compound.
(b) Will this compound produce a clear or sooty flame on burning? Justify your answer.
Answer. (a) 1 mark each for the correct name and formula:
chemical name - Hexane
chemical formula - C6H14
(b) It will burn with a clear flame since it is a saturated hydrocarbon.
-
👉 Read Also- CBSE Class 10 Half-Yearly/Mid Term 2024-25 : Most Important Questions with Answers; PDF Download (All Subjects)
👉 CBSE Class 10 Study Materials
| CBSE Class 10 Syllabus 2024-25 | NCERT Solutions For Class 10 |
| CBSE Class 10 Previous Year Question Papers | CBSE Class 10 Books |
| CBSE Class 10 Full Study Material | CBSE Class 10 Sample Paper |







 Profile
Profile Signout
Signout












 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










