CBSE Class 10th Mid Term Exam 2024-25 : Science Most Important Question with Answers

SHARING IS CARING
If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
Studying for your CBSE Class 10th science mid-term or half-yearly exam can be challenging. To help you out, we’ve put together a list of the most important science questions and answers for the 2024-25 school year. This guide focuses on essential topics from your science syllabus to make studying easier.
👉 Read Also - CBSE Board Class 10th Mid Term Exam 2024-25 : Maths Most Important MCQs with Answers
👉 Read Also - CBSE Board 10th Mid Term Exam 2024-25 : Most Important English Grammar Question with Answers
👉 Read Also - CBSE Board 10th Mid Term Exam 2024-25 : Science (Physics) Most Important Questions with Answers
CBSE Class 10th Mid Term/Half-Yearly Science Questions and Answers
1. You are given the solution of lead nitrate. In order to obtain a yellow precipitate, you should mix it with a solution of:
(a) potassium chloride
(b) potassium nitride
(c) potassium sulphide
(d) potassium iodide
Ans. (d) potassium iodide
2. A solution reacts with crushed egg-shells to give a gas that turns lime-water milky.The solution contains:
(a) NaCl
(b) HCl
(c) LiCl
(d) KCl
Ans. (b) HCl
3. A small electric lamp is placed at the focus of a convex lens. When the lamp is switched on, the lens will produce:
(a) converging beam of light
(b) parallel beam of light
(c) diverging beam of light
(d) diffused beam of light
Ans. (b) parallel beam of light
4. The electronic configurations of three elements X, Y and Z are X : 2, 8; Y : 2, 8, 7 and Z : 2, 8, 2. Which of the following is correct?
(a) X is a metal
(b) Y is a metal
(c) Z is a non-metal
(d) Y is a non-metal and Z is a metal
Ans. (d) Y is a non-metal and Z is a metal
👉 CBSE Class 10 Study Materials
| CBSE Class 10 Syllabus 2024-25 | NCERT Solutions For Class 10 |
| CBSE Class 10 Previous Year Question Papers | CBSE Class 10 Books |
| CBSE Class 10 Full Study Material | CBSE Class 10 Sample Paper |
5. If salivary amylase is lacking in the saliva, which of the following events in the mouth cavity will be affected?
(a) Proteins breaking down into amino acids
(b) Starch breaking down into sugars
(c) Fats breaking down into fatty acids and glycerol
(d) Absorption of vitamins
Ans. (b) Starch breaking down into sugars
6. A person is seeing an object closer to his eyes. What changes in his eyes will take place?
(a) The pupil size will expand
(b) The ciliary muscles will contract
(c) The focal length of eye lens will increase
(d) The light entering in the eye will be more
Ans. (b) The ciliary muscles will contract
7. What is the focal length of a glass slab?
(a) 0
(b) infinite
(c) 1
(d) vary from 0 to 1
Ans. (b) infinite
8. The yellow colour of turmeric changes to red on addition of soap solution. When substance P is added to turmeric, there is no change in colour.
Which of the following is definitely true about substance P?
(a) P is an acid.
(b) P is not a salt.
(c) P is not a base.
(d) P is a neutral substance
Ans. (d) P is a neutral substance
9. Leena was advised by her teacher to wear gloves and use forceps while dipping the pH paper in the liquids.
What was the reason for this advice ?
(I) Gloves keep the hands warm.
(II) Forceps provide better grip than bare hands.
(III) Gloves protect hands from corrosive liquids.
Options:
(a) Only (I)
(b) Only (II)
(c) Only (III)
(d) Both (I) and (II)
Ans. (c) Only (III)
10. In naturopathy, it is suggested that in the morning, we should drink water kept in a copper vessel overnight. However, copper vessel gets tarnished after a few days. It is due to the formation of:
(a) green layer
(b) brown layer
(c) blue layer
(d) red layer
Ans. (a) green layer
11. How does the refractive index of earth’s atmosphere vary with height?
(a) Hotter air is lighter than the cooler air.
(b) Cooler air is lighter than the hotter air.
(c) Refractive index of both air is equal.
(d) Refractive index of upper atmosphere is more than colder layers of atmosphere.
Ans. (a) Hotter air is lighter than the cooler air.
12. In the redox reaction:
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
(a) MnO2 is reduced to MnCl2 and HCl is oxidized to H2O
(b) MnO2 is reduced to MnCl2 and HCl is oxidized to Cl2
(c) MnO2 is oxidized to MnCl2 and HCl is reduced to Cl2
(d) MnO2 is oxidized to MnCl2 and HCl is reduced to H2O
Ans. (b) MnO2 is reduced to MnCl2 and HCl is oxidized to Cl2
13. A ray of light passes through a glass prism.
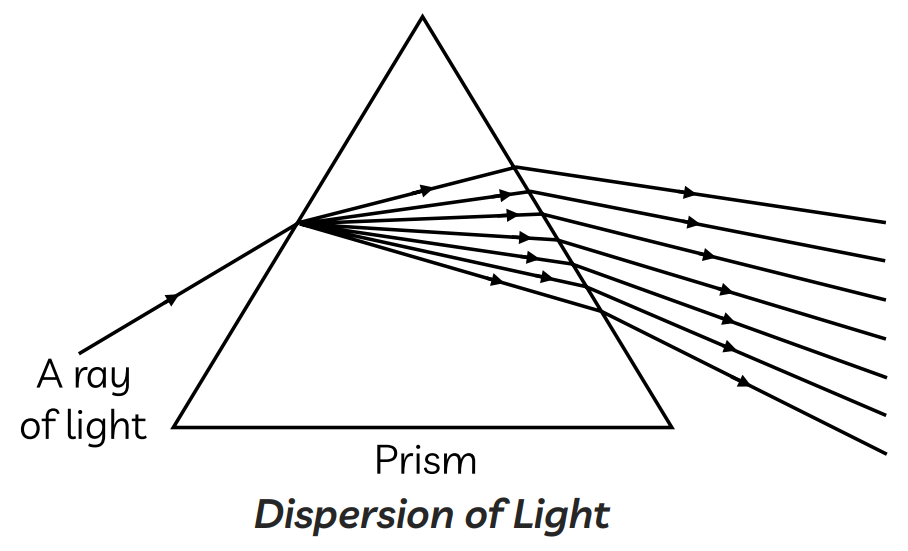
When do the light rays get refracted?
(I) As the light rays enter the prism from the air.
(II) As the light rays travel inside the prism.
(III) As the light rays move from the prism into the air.
Options:
(a) Only (I)
(b) Only (II)
(c) (II) and (III)
(d) (I) and (III)
Ans. (d) (I) and (III)
14. Four combustion reactions of carbon compounds are shown below.
CH4 + O2 → CO2 + H2O + heat
CH3CHO + O → CO2 + H2O + heat
CH3CH2CH2OH + O2 → CO2 + H2O + heat
C6H6 + O2 → CO2 + H2O + heat
What can be concluded from the four reactions?
| (I) | All carbon compounds release oxygen on combustion. |
| (II) | All carbon compounds release water on reacting with oxygen. |
| (III) | All carbon compounds produce carbon dioxide on reacting with oxygen. |
Options:
(a) Only (I)
(b) (I) and (II)
(c) (II) and (III)
(d) (I), (II) and (III)
Ans. (c) (II) and (III)
15 to 18 consist of two statements –
Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, and R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
15. Assertion (A): Metals react with water to form a metal hydroxide or metal oxide and hydrogen gas.
Reason (R): Sodium metal reacts with cold water and form sodium hydroxide and hydrogen gas. Iron reacts with steam to form iron oxide and hydrogen.
Ans. (b) Both A and R are true, and R is not the correct explanation of A.
16. Assertion (A): Lemon pickle can be stored in Aluminium vessel.
Reason (R): Aluminium metal reacts with acid present in lemon and produce hydrogen gas which cause food spoilage.
Ans. (d) A is false but R is true.
17. Assertion (A): A blue-green solution is obtained from a reaction between copper oxide and dilute hydrochloric acid.
Reason (R): This happens due to formation of copper (II) chloride.
Ans. (a) Both A and R are true, and R is the correct explanation of A.
18. Assertion (A): A convex lens has a real focus.
Reason (R): All light rays pass through the focus of a convex lens after refraction
Ans. (c) (A) is true but (R) is false.
19. Oxides of metals are basic while those of non-metals are acidic. Explain.
Ans. Oxides of metals are basic as they react with acids to produce salt and water.
CuO + 2HCl → CuCl2 + H2O
Oxides of non-metals are acidic as they react with bases to produce salt and water.
CO2 + Ca(OH)2 → CaCO3 + H2O
20. The pancreas of a person suddenly stopped functioning. How will digestion be affected in such a person?
Ans. If the pancreas of a person stops functioning, the digestion of starch, fats and proteins will be affected as pancreatic amylase, lipase and trypsinogen secreted by pancreas acting on them respectively will not be secreted anymore.
21. What would have been the colour of sky if the earth had no atmosphere?
Ans. The sky would have looked dark if the earth had no atmoshere as there would not be no scattering.
22. Why do leaves appear green?
Ans. Leaves appear green as they absorb all colours and reflect only the green colour to the eyes.
23. Of the three metals X, Y and Z, X reacts with cold water, Y with hot water and Z with steam only. Identify X, Y and Z and also arrange them in increasing order of reactivity.
Ans. Comparing with the pH chart, we find that orange colour corresponds to pH value of about 4 and blue clour to pH value of about 10.
(1) X is therefore acidic and pH is around 4.
(2) Y is basic having a pH of about 10.
24. An element A burns with a golden flame in air. It reacts with another element B (atomic number 17) to give a product C. An aqueous solution of product C on electrolysis gives a compound D and liberates hydrogen and chlorine gases. Identify A, B, C and D. Also write down the gas equations for the reactions involved.
Ans.
A — Sodium (Na)
B — Chlorine (Cl)
C — Sodium chloride (NaCl)
D — Sodium hydroxide (NaOH)
Element A is sodium that burns with a golden flame.
Element B is chlorine. Its atomic number is 17.
Sodium and chlorine react to form sodium chloride (NaCl) which is an ionic compound.
2Na + Cl2 → 2NaC
the normal and the reflected ray.
Therefore: ∠r = 90° - 70° = 20°
23. Do acidic solutions also have hydroxide ions (OH–1) ions ? Explain with an example.
Ans. Yes, acidic solutions also have hydroxide ions (OH–1) ions besides Hydrogen ions (H–1) ions due to the presence of water. But it is the number of hydroxide ions (OH–1) in water and the number of Hydrogen ions (H+1) ions in water that decides the nature of the solution. In an acidic solution the number of Hydrogen ions (H–1) ions are more than the number of hydroxide ions (OH–1).
For example : HCl + H2O → H+1(aq) + Cl–1(aq) (from the ionisation of Hydrochloric acid) H+1(aq) + OH–1(aq)(from the ionisation of water)Both Hydrogen ions and hydroxide ions are present. The number of Hydrogen ions (H+1) ions are more than the number of hydroxide ions (OH–).
24. Explain the ways in which glucose is broken down in absence or shortage of oxygen.
Ans. The First step in breakdown of the 6-carbon molecule glucose takes place in the cytoplasm of cells of all organism and we obtain a 3-carbon molecule compound cakked pyruvate. Further pathway for breakdown of this pyruvate depends on the amount of oxygen available which are given ahead:
(1) Anaerobic Respiration: It takes place in the absence of oxygen. In this process, the pyruvate is convered into ethanol and carbon dioxide. For example, in yeast during fermentation.
(2) Lack of Oxygen: When ther is a lack of oxygen in our body, the pyruvate is converted into lactic acid, which is a 3-carbon molecule, and energy. This takes place in our muscles during vigorous exercise or activity.
25. (A) State and explain the type of reaction between calcium oxide and water.
Ans. Calcium hydroxide is formed when calcium oxide (quick lime) reacts with water. The reaction is shown below:
CaO(s) + H2O(aq) → Ca (OH)2(s)
(B) State two precautions you should take when reacting calcium oxide with water.
Ans. Precautions to be taken:
(1) Wear goggles.
(2) Use a heat resistant container.
(3) Adding of excessive water than required can cause an explosion owing to a vigorous reaction.
(4) Avoid touching or dipping fingers in the lime water since calcium hydroxide can burn the skin. (Any two)
26. Name the following:
(A) Metal that can be cut by knife
(B) Lustrous non-metal
(C) Metal that exists in liquid state at room temperature
(D) Most malleable and ductile metal
(E) Metal that is best conductor of electricity
(F) Non-metal that can exist in different forms
Ans.
(A) Metal which can be cut by knife: Sodium
(B) Lustrous non-metal: Iodine
(C) Metal that exists in liquid state at room temperature: Mercury
(D) Most malleable and ductile metal: Gold
(E) Metal that is best conductor of electricity: Silver
(F) Non-metal that can exist in different forms: Carbon
27. (A) Write the correct sequence of steps followed during journey of oxygen rich blood from lungs to various organs of human body.
Ans. Journey of oxygen rich blood from lungs to various parts of the body.
(1) The pulmonary vein brings the oxygenated blood from the lungs in the left atrium of the heart.
(2) The left atrium contracts and pumps blood into left ventricle through the valve.
(3) When the left ventricle contracts, the oxygenated blood enters the largest artery aorta.
(4) The blood travels from the aorta to larger and smaller arteries into the capillaries.
(5) The aorta transports the blood to all the organs of the body except the lungs.
(6) The oxygenated blood releases oxygen, nutrients and other substances and takes away carbon dioxide and waste substances.
(7) The deoxygenated blood enters the vena cava which carries it to the right atrium of the heart.
Journey of oxygen rich blood
Lungs
↓ oxygenated blood
Pulmonary vein
↓
Left atrium
↓ contract
Left ventricle
↓ contracts
Aorta
↓
Arteries
↓
All the organs (except lungs)
(B) What happens when the system of blood vessels develop a leak?
Ans. When the system of blood vessels develop a leak, the platelets will increase, which will minimise the leakage. Whenever there is a leak in the blood
vessels the blood flows, through them, If not checked, that may cause an excessive loss of blood.
(1) Process of blood clotting: At the point of leak, the platelets rupture and release a substance thromboplastin.
(2) Thromboplastin converts prothrombin to thrombin.
(3) Thrombin changes soluble fibrinogen protein into fibrin.
(4) Fibrin undergoes rapid polymerization and forms fibers.
(5) Fibers form a network over the injured region, the injured region, entrap blood corpuscles and form a blood clot.
28. The path of light is altered when it moves from one transparent medium to another. This phenomenon is known as light refraction. The optical density of the medium that the light passes through determines how the light will bend.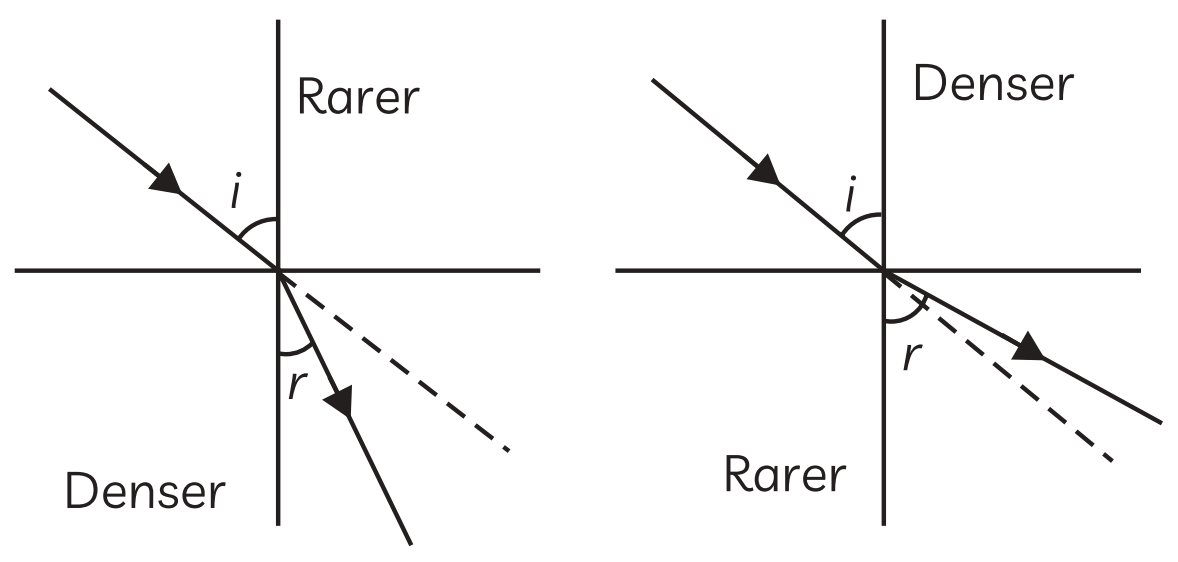 From medium to medium, light travels at varying speeds. A medium with a higher speed of light is optically rarer, whereas a medium
From medium to medium, light travels at varying speeds. A medium with a higher speed of light is optically rarer, whereas a medium
with a lower speed of light is optically denser. When light travels from one medium to another, its frequency does not alter; instead, its speed and wavelength do. It came to the conclusion that the primary cause of refraction is a change in light speed.
(A) How do light rays bend in one of the following ways when they pass through glass from air?
(B) What happens to the frequency of light when it travels from one medium to another?
(C) At what angle the ray of light hits the boundary of medium B so that no bending of light occurs?
Ans.
(A) A light ray bends toward the normal as it passes through glass from air.
(B) When light travels from one medium to another, its frequency remains constant.
(C) When light is incident normally or perpendicularly on a boundary between two media, it does not bend because the angle of incidence and the angle of refraction are both zero.
29. What is the effect on speed when light travels through glass and into water? How do the bottom of a pool filled with water appears due to light refraction?
Ans. As water is an optically rarer medium than glass, light travels faster when it passes through it.
Refraction causes a pool's bottom to appear shallower than it actually is.
30. Which defect is shown in the given figure?
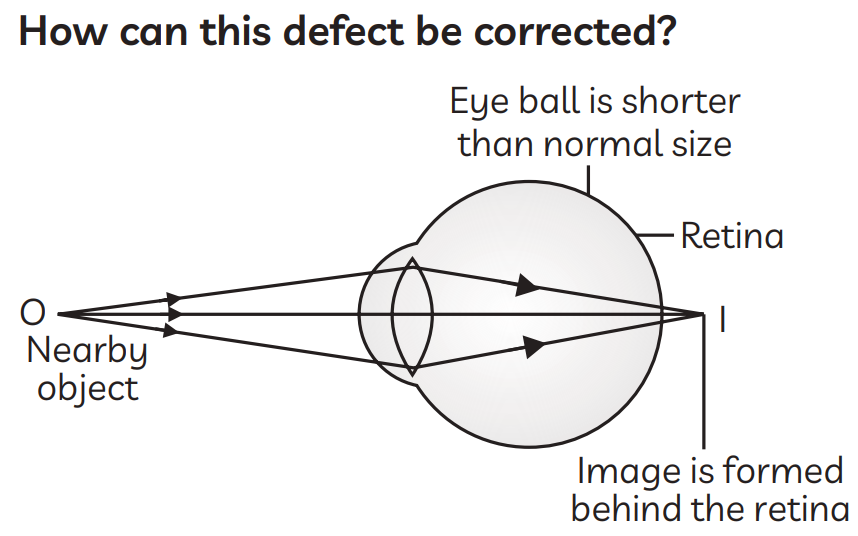
Ans. The defect shown in the given figure is hypermetropia.
Such a person's glasses can be made with a convex lens of a suitable focal length to treat long-sight. The diverging light rays from the nearby object are first brought into convergence by the convex lens when it is placed in front of the hypermetropic eye. As a result, at a location close to the hypermetropic eye, the convex lens creates a virtual image of the object nearby. The convex lens's image is then easily focused on the retina by the hypermetropic eye.







 Profile
Profile Signout
Signout












 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










