Maharashtra Board Chemistry 12th Exam 2024 : Most Important Question Answers for Last-Minute Revision

SHARING IS CARING
If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
Maharashtra Class 12 exams have started and you have very little time left for the Chemistry exam. Therefore, we are providing Most Important Answer Type Questions in this article. You can study them well and score well in your exams.
So this article is to help you ace those Most Important MCQs and Short & Long Question Answers with quick and effective last-minute revision.
MCQs (Multiple Choice Questions) are an important part of your Chemistry exam to score good marks. Mastering them can boost your confidence and lead to important scores. These questions cover various topics from the Chemistry syllabus. Remember, it is important to understand the logic behind each answer to score well.
Read Also -
Maharashtra Board HSC Class 12th 2024 : Physics, Chemistry, Biology Important Questions with Solutions; Download PDF
Maharashtra Board HSC (Class 12) 2024 Chemistry : Practice Paper with Solution; Download PDF
Maharashtra Board Chemistry Most Important Question Answers
Multiple Choice Questions
1. n-type semiconductor is formed when trace amount of impurity is added to silicon. The number of valence electrons in the impurity atom must be.
(a) 3
(b) 5
(c) 1
(d) 2
Ans. (b) 5
2. The number of ions produced by the complex [Co(NH3)4CI2]CI is
(a) 1
(b) 2
(c) 3
(d) 4
Ans. (b) 2
3. The coordination number of atoms in body-centred cubic structure (bec) is
(a) 4
(b) 6
(c) 8
(d) 12
Ans. (c) 8
4. The substances which can be permanently magnetised are
(a) diamagnetic
(b) paramagnetic
(c) ferromagnetic
(d) nonmagnetic
Ans. (c) ferromagnetic
5. In calculating osmotic pressure, the concentration of solute is expressed in
(a) molarity
(b) molality
(c) mole fraction
(d) percentage mass
Ans. (a) molarity
6. The addition of the nonvolatile solute into the pure solvent
(a) increases the vapour pressure of solvent
(b) decreases the boiling point of solvent
(c) decreases the freezing point of solvent
(d) increases the freezing point of solvent
Ans. (c) decreases the freezing point of solvent
7. 10 ml of 0.1 M H2SO4, is mixed with 20 ml of 0.1 M KOH, the pH of resulting solution will be
(a) 0
(b) 7
(c) 2
(d) 9
Ans. (b) 7
8. If the hydrogen ion concentration changes by a factor of 1000, the value of its pH will
(a) decrease by 2 units
(b) decrease by 3 units
(c) increase by 1000 units
(d) increase by 2 units
Ans. (b) decrease by 3 units
9. Which of the following pairs is an intensive property ?
(a) Density, viscosity
(b) Surface tension, mass
(c) Viscosity, internal energy
(d) Heat capacity, volume
Ans. (a) Density, viscosity
10. The enthalpy of formation for all elements in their standard states is
(a) unity
(b) zero
(c) less than zero
(d) different elements
Ans. (b) zero
11. Two solutions have the ratio of their concentrations 0.4 and ratio of their conductivities 0.216. The ratio of their molar conductivities will be
(a) 0.54
(b) 11.574
(c) 0.0864
(d) 1.852
Ans. (a) 0.54
12. During the discharging of a lead storage battery,
(a) H2SO4, is consumed
(b) PbSO4 is consumed
(c) Pb2+ ions are formed
(d) Pb is formed
Ans. (a) H2SO4, is consumed
13. The units of rate of a reaction and rate constant are same for a reaction of order
(a) zero
(b) one
(c) two
(d) fractional
Ans. (a) zero
14. The half-life of a first order reaction is 30 min and the initial concentration of the reactant is 0.1 M. If the initial concentration of reactant is doubled, then the half-life of the reaction will be
(a) 1800 s
(b) 60 min
(c) 15 min
(d) 900 s
Ans. (a) 1800 s
15. Among the known interhalogen compounds, the maximum number of atoms present is
(a) 3
(b) 6
(c) 7
(d) 8
Ans. (d) 8
16. The oxidation number of Xe in XeOF2 is
(a) 0
(b) +2
(c) +4
(d) +3
Ans. (c) +4
17. Which one of the following elements belong to actinoid series?
(a) Cerium
(b) Lutetium
(c) Thorium
(d) Lanthanum
Ans. (c) Thorium
18. The total number of elements in each of f-series is
(a) 10
(b) 12
(c) 14
(d) 15
Ans. (c) 14
19. Magnetic moment of a metal complex is 5.9 B.M. Number of unpaired electrons in the complex is
(a) 2
(b) 3
(c) 4
(d) 5
Ans. (d) 5
20. Highest magnetic moment is shown by the ion
(a) V3+
(b) Co3+
(c) Fe3+
(d) Cr3+
Ans. (c) Fe3+
21. The number of unpaired electrons in a low spin octahedral complex ion of d' is
(a) 0
(b) 1
(c) 2
(d) 3
Ans. (b) 1
22. Ligand used in the estimation of hardness of water is
(a) EDTA
(b) DBG
(c) chloride
(d) bromo
Ans. (a) EDTA
23. Precipitation of protein is referred to as
(a) destruction of proteins
(b) separation of proteins
(c) denaturation of proteins
(d) fragmentation of proteins
Ans. (c) denaturation of proteins
24. Terylene is
(a) polyamide fibre
(b) polyester fibre
(c) vegetable fibre
(d) protein fibre
Ans. (b) polyester fibre
25. The constituents of carbon nanotubes are
(a) nanosized graphite sheets
(b) nanosized carbon black
(c) nanosized coal black
(d) None of the above
Ans. (a) nanosized graphite sheets
Very Short Answer Questions
Q1. What is valence band ?
Ans. Valence bond is a partially or completely filled band produced by bonding MOS, from which electrons can be promoted to conduction band.
Q2. What are ferromagnetic substances ?
Ans. The substances containing large number of unpaired electrons are strongly attracted by magnetic fields. These substances are called ferromagnetic substances.
Q3. What are hypotonic solutions?
Ans. Hypotonic solutions: When two solutions have differen osmotic pressures, then the solution having lower osmotic pressure is said to be a hypotonic solution with respect to the other solution.
Q4. Give an example of an ideal solution.
Ans. A liquid mixture of benzene and toluene which have nearly identical physical properties and intermolecular forces forms an ideal solution.
Q5. What is meant by conjugate acid-base pair ?
Ans. A pair of an acid and a base differing by a proton is called conjugate acid-base pair. For example, HCl and Cl-.
Q6. What is common ion effect ?
Ans. The ionization of a weak electrolyte is suppressed in presence of a strong electrolyte containing an ion common to the weak electrolyte.
Q7. What is thermodynamic equilibrium ?
Ans. A system is said to be in thermodynamic equilibrium when its state functions do not vary with time.
Q8. What is an intensive property ? Give an example.
Ans. Intensive property : It is a property of a system whose magnitude is independent of the amount of matter present in the system. For example, viscosity.
Q9. What are the functions of salt bridge ?
Ans. The functions of a salt bridge are :
(1) It maintains the electrical contact between the two electrod solutions of the half cells.
(2) It prevents the mixing of electrode solutions.
(3) It maintains the electrical neutrality in both the solutions of two half cells by a flow of ions.
(4) It eliminates the liquid junction potential.
Q10. What is conductivity ?
Ans. The reciprocal of resistivity is called conductivity. It is the conductance of a conductor of unit length and unit cross section area. OR It is the conductance of unit cube of material.
Q11. What is the rate constant of a reaction ?
Ans. Rate constant of the reaction is its rate when concentrations or partial pressures of all the reactants is unity.
Q12. What is the rate determining step of a chemical reaction ?
Ans. It is the slowest step in the mechanism of a complex reaction that occurs in more than one step.
Q13. What is the reaction intermediate in a complex reaction ?
Ans. The reaction intermediate is a species other than reactants or products produced in one step of the mechanism of a complex reaction and consumed in the subsequent step.
Q14. What are pseudo first order reactions ?
Ans. The reactions that have higher order true rate law but follow first order kinetics are called pseudo first order reactions.
Q15. What is hydrometallurgy ?
Ans. Hydrometallurgy : It is a process of extraction of metals from the aqueous solutions of their salts using suitable reducing agents.
Q16. Name an ether which is free from moisture and alcohol.
Ans. The ether which is free from moisture and alcohol is absolute ether.
Q17. Name a compound which has similar geometry to ether.
Ans. A compound which has similar geometry to ether is water.
Q18. State the constituents of natalite.
Ans. The constituents of natalite are diethyl ether and ethanol.
Q19. Which of the following is more volatile : o-nitrophenol or p-nitrophenol ?
Ans. The isomer o-nitrophenol with lower boiling point is more volatile.
Q20. Why does skin have burning sensation, when an ant bites ?
Ans. When an ant bites, formic acid is released from an ant which gives burning sensation as the acid comes in contact with the skin.
Q21. What is the percentage of acetic acid in vinegar ?
Ans. The percentage of acetic acid in vinegar is 6 to 8%.
Q22. What is formalin ?
Ans. The aqueous solution of formaldehyde (40%) is known as formalin.
Q23. Write the IUPAC name of benzyl amine.
Ans. The IUPAC name of benzyl amine is Phenylmethanamine.
Q24. Name the sugar present in RNA.
Ans. The sugar present in RNA is D-ribose.
Q25. What are the disadvantages of nanotechnology ?
Ans. Nano pollution and lung damage.
Defination
1. Coordination number.
Ans. The number of the closest neighbouring constituent particles like atoms, ions or molecules which are in contact with a particular particle or an atom in the crystal lattice is called coordination number of that particle.
2. Osmosis.
Ans. It is defined as a spontaneous net flow of the solvent molecules from a pure solvent to a solution or from a more dilute solution to the more concentrated solution through a semipermeable membrane.
3. Buffer solution.
Ans. It is defined as a solution which resists the change in pH even after the addition of a small amount of a strong acid or a strong base or on addition of water.
4. Closed system.
Ans. It is defined as a system which can exchange only energy but not the matter with its surroundings.
5. Standard electrode potential.
Ans. It is defined as the difference of electrical potential between metal electrode and the solution around it at equilibrium when all the substances involved in the electrode reaction are in their standard states of unit activity or concentration at constant temperature.
6. Energy of activation.
Ans. It is the minimum kinetic energy possessed by the colliding reactant molecules leading to the formation of the products of the reaction
7. Coordination compound.
Ans. It is a compound in which central metal ion or atom is bound to surrounding atoms, molecules or anions called ligands by coordinate bonds.
8. Coordination sphere.
Ans. A coordination entity consisting of a central metal atom or ion and the ligands enclosed inside a square bracket is called coordination sphere.
9. Ligands.
Ans. The neutral molecules or negatively charged ions (or rarely positive ions) which are bonded by coordinate bonds to the central metal atom or metal ion in a coordination compound are called ligands.
10. Plane polarized light.
Ans. A light having oscillations only in one plane perpendicular to the direction of propagation of light is known as plane polarized light.
11. Chiral carbon atom.
Ans. A carbon which is attached to four different atoms or groups is called chiral carbon atom.
12. Nucleotide.
Ans. Nucleotide are monophosphates of nucleosides. It contains all three basic components of nucleic acids, i.e. a pentose sugar, a phosphoric acid and a nitrogeneous base.
13. Elastomers.
Ans. Polymers in which the intermolecular forces of attraction between the polymer chains are the weakest. When polymer is stretched, it has ability to stretch and when the strain is relieved it returns to its original position.
14. Vulcanization.
Ans. The process by which a network of cross links is introduced into an elastomer is called vulcanization.
15. Nanotechnology.
Ans. The design, characterization, production and application of structures, device and system by controlling shape and size at nanometer scale is called nanotechnology.
Laws & Theory
1. State the second law of thermodynamics in terms of entropy.
Ans. The second law of thermodynamics states that the total entropy of the system and its surroundings (universe) increases in a spontaneous process.
2. Explain the steps involved in the metal-ligand bonding.
Ans.
(1) Find the oxidation state of central metal ion in the complex. Write the electronic configuration of free metal ion, and display orbital diagram of valence shell electron configuration of metal ion.
(2) Decide the type of hybridization from the geometry of 4 coordinate complex and from high/low spin nature of octahedral complex.
(3) In order to make orbitals vacant that are required for hybridization, carry out forced pairing and/or transfer of electrons. If necessary, display the orbital diagram accordingly.
(4) Name the orbitals involved in hybridization.
(5) Mention the overlapping of required orbitals. Sketch the orbital diagram with shared pair of electrons.
3. State and explain Henry's law.
Ans. Statement of Henry's law : It states that the solubility of a gas in water at constant temperature is proportional to the pressure of the gas above the solution. S ∝ P or S = KHP, where S is the solubility of gas, P its pressure. KH the proportionality constant is called Henry's law constant.
4. State and explain Raoult's law.
Ans. Statement of Raoult's law : The law states that, at constant temperature, the partial vapour pressure of any volatile component of a solution is equal to the product of vapour pressure of the pure component and the mole fraction of that component in the solution. For mixture of two liquids, A and B, PA = P0AxA, PB = P0BxB, where PA and PB are their vapours and ХА and XB are their mole fractions in the mixture. P0A and P0B are the mole fractions of pure liquids A and B respectively.
5. Aldehydes give positive test for Fehling's solution while ketones give negative test.
Ans. Aldehydes can be oxidised to their corresponding carboxylic acids by Fehling's solution but ketones cannot be oxidised hence ketones give negative test for Fehling's solution.
6. Explain the rate law with example. Give illustrations.
Ans. Explanation : Consider the following reaction
aA + bB → products
The rate of the reaction at a given time is proportional to the molar concentrations of reactants at that time raised to simple powers.
R ∝ [A]x [B]y
∴ R = k [A]x [B]y,
where [A] = concentration of A and [B] = concentration of B at the given time.
The proportionality constant k is called the velocity constant, rate constant or specific rate of the reaction.
x and y are the exponents or the powers of the concentrations of the reactants A and B respectively when observed experimentally.
Example :
H2(g) + I2(g) → 2HI(g)
R = k[H2] [I2].
7. What are the salient features of valence bond theory (VBT) ?
Ans. The salient features of valence bond theory (VBT) are as follows :
(1) VBT describes the process of hybridization and thereby bonding in metal complexes.
(2) According to this theory, a central metal atom or ion present in a complex has a definite number of vacant orbitals (s, p, d and f) equal in number to its coordination number.
(3) The vacant orbitals of metal atom or ion undergo hybridization forming the same number of vacant hybrid orbitals. Under the influence of ligands, metal ion/atom uses ns, np and (n-1)d or nd orbitals for hybridization.
(4) If (n-1)d orbitals are used by metal ion for hybridization, the complex is called inner orbital or low spin complex. If nd orbitals are used the complex is called outer orbital or high spin complex.
(5) Each ligand has at least one orbital that contains a lone pair of electrons. The vacant hybrid orbitals of metal accept a share of electrons from ligands by overlapping with filled orbitals of ligands. This results in the formation of metal-ligand coordinate bonds.
(6) VBT description of bonding in complexes requires
(i) geometry, tetrahedral or square planar or magnetic nature for 4 coordinate complexes
(ii) high spin or low spin nature (or magnetic behaviour) of octahedral complexes with d4 to d7 electron configurations.
8. Define acids and bases on the basis of Lewis concept. Give examples.
Ans. Lewis concept of an acid and a base is based on the electronic theory.
Acid : It is defined as any species (molecule or ion) that can accept a pair of electrons. E.g. BF3, AlCl3 and all electron deficient species like cations (K+, Ag) and molecules having incomplete octet, like BeF2, BF3.
Base : It is defined as any species (molecule or ion) that can donate a pair of electrons. E.g. NH3, C2H5NH2 and all electron rich species like anions (CI-, OH-) and all molecules with lone pair of electrons.
Short Answer Questions
Q1. What are Bravais lattices ?
Ans. (1) There are seven crystal systems according to the edges (a, b, c) and angles (α, β, γ).
(2) The constituents of the crystal may be present at corners, face centres, body centres, edge centres and voids.
(3) By mathematical analysis, it has been proved that only fourteen different kinds of space lattices are possible.
(4) Hence there are fourteen different ways of arrangement of the lattice basis.
(5) These fourteen lattices of seven crystal systems are called Bravais lattices.
Q2. What are the types of unit cells ?
Ans. Basically unit cells are of two types as follows :
(1) Primitive unit cell : The unit cell in which the constituent particles like atoms, ions or molecules are present only at the corners of the unit cell is called primitive unit cell or simple unit cell.
(2) Body-centred unit cell : A unit cell in which the constituent particles are present at the corners as well as at its body-centre is called body-centred unit cell.
(3) Face-centred unit cell : A unit cell in which the constituent particles are present at the corners as well as at the centre of each face is called face-centred unit cell or cubic close packed (CCP) unit cell.
(4) Base-centred unit cell : A unit cell in which the constituent particles are present at the corners as well as at the centres of two opposite faces is called end-centred or base centred unit cell.
Q3. What are semiconductors ? Explain intrinsic and extrinsic semiconductors.
Ans. Semiconductors : The substances like silicon, germanium which have poor electrical conductance at low temperature but the conductance increases with the increase in temperature are called semiconductors. Their conductivity lies between metallic conductors and insulators.
(a) Intrinsic semiconductor :
(i) A pure semiconductor material like pure Si, Ge which have a very low but finite electrical conductivity is called intrinsic semiconductor.
(b) Extrinsic semiconductor :
(i) Semiconductor doped with different element is called extrinsic semiconductor.
(ii) By doping with elements like Ga or P, the electrical conductivity is increased.
Q4. Explain the terms :
(1) Isotonic solutions
(2) (i) Hypotonic solutions (ii) Hypertonic solutions.
Ans.
(1) Isotonic solutions : The solutions having the same osmotic pressure at a given temperature are called isotonic solutions.
Explanation :
(i) For example, 0.1M urea solution and 0.1M sucrose solution are isotonic because they have the same osmotic pressure.
(ii) Isotonic solutions have the same molar concentration.
(iii) If isotonic solutions are separated by a semipermeable membrane, there is no flow of solvent in either direction.
(2) (i) Hypotonic solutions: When two solutions have different osmotic pressures, then the solution having lower osmotic pressure is said to be a hypotonic solution with respect to the other solution.
(ii) Hypertonic solutions: When two solutions have different osmotic pressures, then the solution having higher osmotic pressure is said to be a hypertonic solution with respect to the other solution. For example, if the osmotic pressure of sucrose solution is higher than that of urea solution then
(i) urea solution is hypotonic to the sucrose solution and
(ii) sucrose solution is hypertonic to urea solution.
Q5. Define buffer solution. What is buffer solution ? What are the types of buffer solution ?
Ans. Buffer solution : It is defined as a solution which resists the change in pH even after the addition of a small amount of a strong acid or a strong base or on dilution or on addition of water.
These are two types of buffer solution :
(A) Acidic buffer solution : It is a solution containing a weak acid (e.g. CH3COOH) and its salt of a strong base. (e.g. CH3COONa).
(B) Basic buffer solution : It is a solution containing a weak base (e.g. NH4OH) and its salt of strong acid (e.g. NH4CI).
Q6. Define molar conductivity. What is the significance of it ?
Ans. Molar conductivity : It is defined as a conductance of a volume of the solution containing ions from one mole of an electrolyte when placed between two parallel plate electrodes 1 cm apart and of large area, sufficient to accommodate the whole solution between them, at constant temperature. It is denoted by ∧m.
Thus, the significance of molar conductivity is the conductance due to ions from one mole of an electrolyte.
Q7. Define Rate law.
Write the rate law for the following reactions :
(a) A reaction that is zero order in A and second order in B.
(b) A reaction that is second order in NO and first order in Br2.
Ans. Rate law : It is defined as an experimentally determined equation which expresses the rate of a chemical reaction in terms of molar concentrations of the reactants raised to simple powers.
For example, for a reaction, aA + bB → Products
By rate law, Rate = R = k[A]x [B]y,
where k is a rate constant and [A] and [B] are molar concentrations of the reactants A and B respectively.
(a) Given : A + B → Products
The reaction is zero order in A and second order in B. Hence, the rate law is represented as,
Rate = k [A]0 [B]2
∴ Rate = k [B]2
(b) Given : 2NO(g) + Br2(g) → 2NOBr(g)
The reaction is second order in NO and first in Br2. Hence, the rate law is
∴ Rate = k [NO2][Br2]
(a) Rate law : Rate = k [B]2,
(b) Rate law : Rate = k [NO]2(Br2].
Q8. The rate law for the reaction
2H2(g) + 2NO(g) → N2(g) + 2H2O(g)
is given by rate = k[H2][NO]2.
The reaction occurs in the following two steps :
(a) H2(g) + 2NO(g) → N2O(g) + H2O(g)
(b) N2O(g) + H2(g) → N2(g) + H2O(g)
What is the role of N2O in the mechanism ? What is the molecularity of each of the elementary steps ?
Ans. N2O is a reaction intermediate which is formed in the first step and removed in the second step.
Molecularity of the elementary steps :
Step 1 : Termolecular.
Step 2 : Bimolecular.
Q9. Define and explain the term elementary reaction.
Ans. Elementary reaction: It is defined as the reaction which takes place in a single step and cannot be divided further into simpler chemical reactions.
The order and molecularity of the elementary reaction are same.
For example,
C2H5I(g) → C2H4(g) + HI(g)
O3(g) → O2(g) + O(g)
Q10. Chemical reaction occurs in the following steps :
(i) NO2(g) + F2(g) → NO2F(g) + F(g) (Slow)
(ii) F(g) + NO2(g) → NO2F(g) (Fast)
(a) Write the equation of overall reaction.
(b) Write down rate law.
(c) Identify the reaction intermediate.
Ans.
(a) Overall reaction : 2NO2(g) + F2(g) → 2NO2F(g)
(b) Rate law : Rate = k[NO2] x [F2]
(c) Reaction intermediate is F(g), as it is formed in step (i) and consumed in step (ii).
Q11. Write similarities between lanthanoids and actinoids ?
Ans. Lanthanoids and actinoids show similarities as follows :
(1) Both, lanthanoids and actinoids show +3 oxidation state.
(2) In both the series, the f-orbitals are filled gradually.
(3) Ionic radius of the elements in both the series decreases with increase in atomic number.
(4) Electronegativity in both the series is low for all the elements.
(5) They all are highly reactive.
(6) The nitrates, perchlorates and sulphates of all elements are soluble while their hydroxides, fluorides and carbonates are insoluble.
Q12. Name the reagents used to convert phenol into (1) picric acid (2) 2,4,6-tribromophenol (3) benzene (4) o-phenol sulphonic acid.
Ans.
(1) The reagent used to convert phenol into picric acid is concentrated nitric acid.
(2) The reagent used to convert phenol into 2,4,6-tribromophenol is bromine water.
(3) The reagent used to convert phenol into benzene is zinc dust.
(4) The reagent used to convert phenol into o-phenol sulphonic acid is dilute sulphuric acid.
Q13. What is the effect of denaturation on the structure of proteins ?
Ans. Proteins gets easily precipitated. It is an irreversible change and the process is called denaturation of proteins. Denaturation uncoils the protein and destroys the shape and thus loses their characteristic biological activity. Denaturation is brought about by heating the protein with alcohol, concentrated inorganic acids or by salts of heavy metals. During denaturation secondary, tertiary and quaternary structures are destroyed but primary structure remains intact. Example: Boiling of egg to coagulate egg white, conversion of milk to curd.
Q14. Explain vulcanization of rubber. Which vulcanizing agents are used for the following synthetic rubber ? 1. Neoprene, 2. Buna-N.
Ans. The process by which a network of cross links is introduced into an elastomer is called vulcanization.
Vulcanization enhances the properties of natural rubber like tensile strength, stiffness, elasticity, toughness, etc. Sulphur forms cross links between polyisoprene chains which results in improved properties of rubber.
(1) For neoprene vulcanizing agent is MgO.
(2) For Buna-N vulcanizing agent is sulphur.
Q15. How nanotechnology plays an important role in water purification techniques ?
Ans. (1) Water purification is an important issue as 1.1 billion people do not have access to improved water supply. Water contains water-bome pathogens like viruses, bacteria.
(2) Silver nanoparticles are highly effective bacterial disinfectant to remove E-coli from water. Hence, filter materials coated with silver nanoparticles is used to clean water. Silver nanoparticles (AgNps) is a cost- effective alternative technology (for e.g. water purifier).
Long Answer Question
Q1. Describe briefly the stacking of spheres in different layers to form three dimensional square close packed lattice.
Ans. (i) First layer (A layer) : In first layer, various one dimensional rows are placed on one over other so that each sphere in one row is over the another sphere of another row forming planar structure. In this, spheres have horizontal as well as vertical alignment.
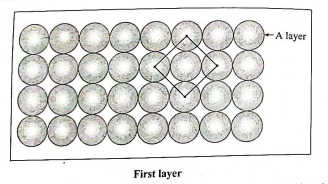
(ii) Second layer : A second layer of spheres can be added by placing spheres directly above those of first layer. The second layer being identical to the first layer is called A layer.
(iii) Similarly, more layers of spheres can be added in such a way that the spheres in one layer are sitting directly on top of those in the previous layer.
(iv) All the layers being identical are labelled as A layers. The pattem therefore is identified as AAAA ... pattern. The unit cell of this lattice is simple cubic. The coordination number of each atom is 6.
Q2. What are point defects ? What are its types ? Explain vacancy defect.
Ans. Point defects : These defects arise due to irregularities produced in the arrangement of lattice points in crystalline solids.
Types of point defects : There are three major classes of point defects: stoichiometric point defects, impurity defects and nonstoichiometric point defects.
Vacancy defect :
(1) During crystallization, some of regular sites in solid remain unoccupied. The missing particle creates a vacancy defect.
(2) The defect can be developed by heating the substance.
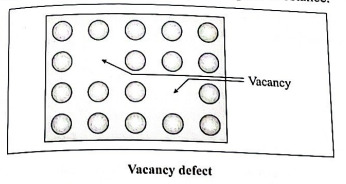
(3) The mass of solid decreases due to absence of particles in regular sites.
(4) Since the volume remains the same the density of the substance decreases.
Q3. Explain the anomalous properties of fluorine.
Ans. The anomalous properties of fluorine are as follows :
(1) Fluorine has the highest reactivity among other halogens.
(2) Fluorine forms strong hydrogen bonding in its hydrides unlike other halogens.
(3) HF is a liquid while other hydrogen halides are gases at room temperature.
(4) HF is a weak acid while other haloacids are strong acids.
(5) Fluorine shows only one oxidation state -1 while all other halogens show variable oxidation states like -1, +1, +3, +5 and +7.
(6) Fluorine has the highest electronegativity but less negative electron gain enthalpy than chlorine.
Q4. What are the properties of lanthanoids ?
Ans.
(1) Lanthanoids are soft metals with silvery white colour. Colour and brightness reduces on exposure to air.
(2) They are good conductors of heat and electricity.
(3) Except promethium (Pm), all are nonradioactive in nature.
(4) The atomic and ionic radii decrease from La to Lu. (Lanthanoid contraction).
(5) They are paramagnetic.
(6) All lanthanoids form hydroxides which are ionic and basic. Basicity decreases with atomic number.
Q5. Explain chemical composition of nucleic acids.
Ans. Nucleic acids have a polynucleotide structure. Nucleic acids (RNA and DNA) consists of three components : (1) monosaccharide (sugar), (2) nitrogen containing base and (3) phosphate group.
(1) Monosaccharides : Nucleotides of both RNA consist of five membered monosaccharide ring (furanose), called as simply sugar component.
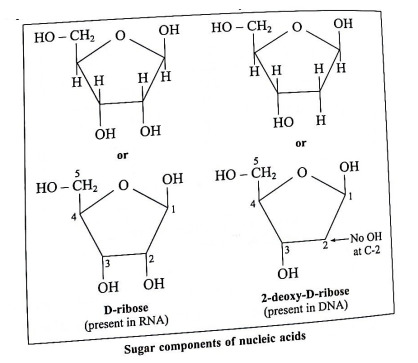
In RNA, the sugar component of nucleotide units is D-ribose and in DNA 2-deoxy-D-ribose.
2-Deoxy means no - OH group at C2 position.
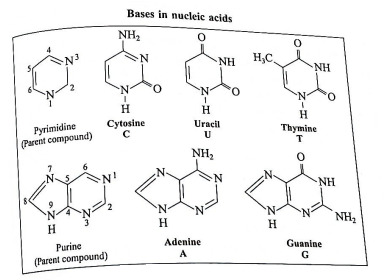
(2) Nitrogen containing base : Total five nitrogen containing bases are present in nucleic acids. Three bases with one ring (cytosine, uracil and thymine) are derived from the parent compound pyrimidine. Two bases with two rings (adenine and guanine) are derived from the parent compound purine. Each base is designated by a one-letter symbol. Uracil (U) occurs only in RNA while thymine (T) occurs only in DNA.
(3) Phosphate group : The sugar units are joined to phosphate through C3 and C5 hydroxyl groups.
Q6. (a) Find the number of atoms per bcc unit cell.
(b) Calculate the packing efficiency of metal crystal that has simple cubic structure.
Ans. (a) Number of atoms in body-centred cubic (bec) unit cell :
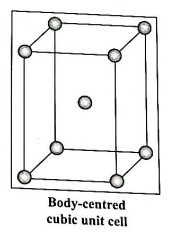
In this unit cell, there are 8 atoms at 8 corners and one additional atom at the body centre. Each corner contributes 1/8th atom, to the unit cell, hence due to 8 corners.
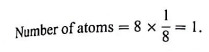
An atom at the body centre wholly belongs to the unit cell.
∴ total number of atoms present in bcc unit cell = 1 + 1 = 2.
Hence the volume of unit cell is equal to the volume of two atoms.
(b) Step 1 : Radius of sphere: In simple cubic lattice, the atoms (spheres) are present at eight corners and in contact along the edge in the unit cell.
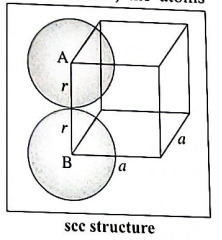
If 'a' is the edge length of the unit cell and 'r' is the radius of the atom, then
a = 2r or r = a/2.
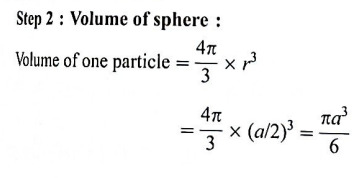
Step 3 : Total volume of particles: Since the unit cell contains one particle, volume occupied by one particle in 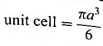
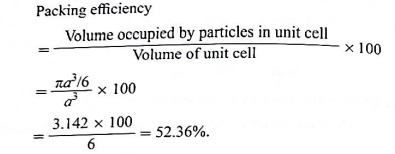
Step 4 : Packing efficiency :
Q7. Name the seven crystal systems. Explain the unit cells of cubic system.
Ans. The seven crystal systems are named as :
(a) Cubic system
(b) Tetragonal system
(c) Orthorhombic system
(d) Rhombohedral system
(e) Monoclinic system
(f) Triclinic system
(g) Hexagonal system.
Cubic lattice : For this, edges are a = b = c and angles are x - y = 90°. In this cubic system, there are three Bravais lattices.
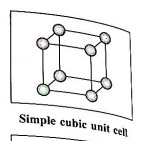
(1) Simple (or primitive) cubic unit cell (SCC) : In this unit cell, atoms are present only at 8 corners of the cube.
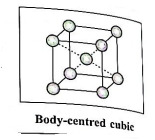
(2) Body-centred cubic unit cell (BCC) : In this, atoms are present at 8 corners along with one additional atom at the body-centre of the cube.
(3) Face-centred cubic unit cell (FCC) : In this unit cell, atoms are present at 8 corners and at 6 face centres.
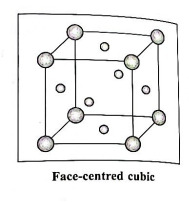
Q8. Predict the sign of ΔS in the following processes. Give reasons for your answer :
(a) CO2(g) → CO2(s)
(b) Fe2O3(s) + 3H2(g) → 2Fe(s) + 3H2O(g)
(c) Cl2(g) → 2Cl(g)
(d) MgCO3(s) → MgO(s) + CO2(g).
Ans. (a) CO2(g) → CO2(s)
In this system from higher disorder in gaseous state changes to less disorder in the solid state, hence entropy decreases, ΔS < 0 or negative.
(b) Fe2O3(s) + 3H2(g) → 2Fe(s) + 3H2O(g)
In the reaction number of moles of gaseous reactants and products are same, hence ΔS = 0.
(c) Cl2(g) → 2Cl(g)
Since the dissociation of Cl2 gas gives two Cl atoms, the number of gaseous atoms increases, increasing the disorder of the system. Hence ΔS > O or positive.
(d) MgCO3(s) → MgO(s) + CO2(g)
In this 1 mole of orderly solid MgCO3 gives 1 mole of solid MgO and 1 mole of gaseous CO2. Molecular disorder increases. Hence entropy increases, ΔS > 0.
Q9. Define and explain :
(A) Enthalpy of atomization
(B) Enthalpy of ionization.
Ans. (A) Enthalpy of atomization (ΔatoH): The enthalpy change or amount of heat absorbed accompanying the dissociation of the molecules in one mole of a gaseous substance into free gaseous atoms at constant temperature and pressure is called enthalpy of atomization.
For example,
Cl2(g) → 2Cl(g)
ΔatoH = 242 kJ mol-1
CH4(g) → C(g) + 4H(g)
ΔatoH = 1660 kJ mol-1
(B) Enthalpy of ionization (ΔionH) : The enthalpy change or amount of heat absorbed accompanying the removal of one electron from each atom or ion in one mole of gaseous atoms or ions is called enthalpy of ionization.
For example,
Na(g) → Na*(g) + e-
ΔionH = 494 kJ mol-1
This equation describes that when one mole of gaseous sodium atoms, Na(g) are ionized forming gaseous ions, Na'(g), the energy required is 494 kJ.
Q10. Discuss the spontaneity of reactions in terms of signs of ΔH and ΔS.
Ans.
(1) ΔG = ΔH - TΔS. ΔG is negative for spontaneous process.
(2) If ΔH and ΔS are both negative, then ΔG is given by ΔG = -ΔH +TΔS. So, ΔG will be negative only when TΔS < ΔH or when temperatures T is low. Such reactions are spontaneous at low temperatures.
(3) If ΔH and ΔS are both positive, then ΔG is given by ΔG = ΔH - TΔS, ΔG will be negative if, TΔS > ΔH; or 7 must be high.
Such reactions are spontaneous at high temperatures.
(4) If ΔH is negative and ΔS is positive, then for all temperatures ΔG is given by ΔG = ΔH -TΔS. ΔG will be negative at all temperatures. But such reactions are spontaneous at all temperatures.
(5) If ΔH is positive and ΔS is negative, ΔG = ΔH + TΔS. Therefore, ΔG will be always positive and hence such reactions are non-spontaneous a all temperatures.
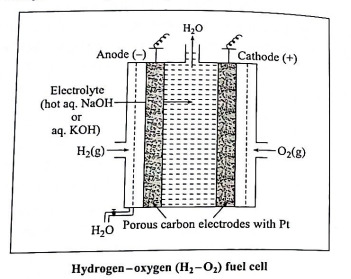
Q11. Describe the construction of H2 - O2 fuel cell. Write electrode reactions and net cell reaction.
Ans. Construction :
(1) In fuel cell the anode and cathode are porous electrodes with suitable catalyst like finely divided platinum.
Q12. Define : Enantiomers. Explain optical isomerism in 2-chlorobutane.
Ans. Enantiomers : The optical isomers which are non-superimposable mirror images of each other are called enantiomers.
Example : 2-Chlorobutane.
(1) 2-Chlorobutane contains an asymmetric.
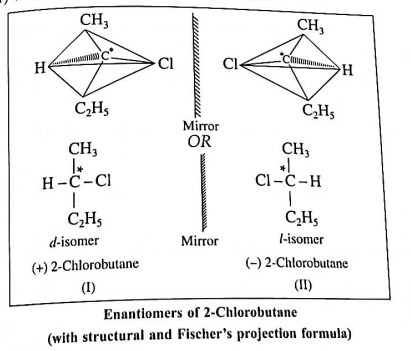
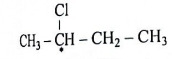 carbon atom (the starred carbon atom) which is attached to four different groups, i.e. ethyl (-CH2 - CH3), methyl (CH3), chloro (Cl) and hydrogen (H) groups.
carbon atom (the starred carbon atom) which is attached to four different groups, i.e. ethyl (-CH2 - CH3), methyl (CH3), chloro (Cl) and hydrogen (H) groups.
(2) Two different arrangements of these groups around the carbon atom are possible as shown in the figure. Hence, it exists as a pair of enantiomers. The two enantiomers are mirror images of each other and are not superimposable.
(3) One of the enantiomers will rotate the plane of plane-polarized light to the left hand side and is called the laevorotatory isomer (l-isomer). The other enantiomer will rotate the plane of plane-polarized light to the right hand side and is called the dextrorotatory isomer (d-isomer).
(4) Equimolar mixture of the d- and the l-isomers is optically inactive and is called the racemic mixture or the racemate (dl-mixture). The optical inactivity of the racemic mixture is due to external compensation.
Remember:
- These are just some of the important topics and strategies. Make sure to revise your entire syllabus according to the weightage mentioned in the official syllabus.
- Don't panic during the exam. Read the questions carefully, manage your time effectively, and answer to the best of your ability.
Good luck with your exams!
Read Also -
Maharashtra Board HSC (Class 12) 2024 Chemistry : Practice Paper with Solution; Download PDF
Maharashtra Board HSC (Class 12) 2024 Biology : Practice Paper with Solution; Download PDF
Maharashtra Board HSC (Class 12) 2024 Physics : Practice Paper with Solution; Download PDF
Maharashtra Board HSC (Class 12) 2024 Mathematics : Practice Paper with Solution; Download PDF
--
Maharashtra Board Class 12 Study Material
| Maharashtra Board Class 12 Study Material | |
| Syllabus | Maharashtra Board New Books |
| Model Paper | Revision Notes |
| Maharashtra Board Previous Year Paper | |







 Profile
Profile Signout
Signout












 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










