JEE Advanced Sample Paper
Preparing for the Joint Entrance Examination (JEE) Advanced can be a challenging yet rewarding journey for aspiring engineers across the nation. As one of India's most prestigious engineering entrance exams, it opens doors to the country’s premier engineering institutes, including the esteemed Indian Institutes of Technology (IITs). You can navigate through this competitive exam with confidence and competence by using a valuable resource known as JEE Advanced sample papers from the SelfStudys website.
On our platform, JEE Advanced sample papers with solutions PDF are carefully crafted to mirror the actual exam pattern, question types, and difficulty level. They provide you with a realistic environment for the examination. From problem-solving skills and analytical thinking to time management and exam strategy, you can sharpen your abilities through regular practice with these papers.
Format Of JEE Advanced Sample Paper On SelfStuyds
There are a number of JEE Advanced Entrance Exam sample papers available on our website for free practice. The scheme or format of these papers is discussed in the table below.
|
PARTICULARS |
DETAILS |
|
Type of Sample Paper |
JEE Advanced sample papers with solutions PDF |
|
Time Allowed to Solve the Sample Paper |
180 minutes |
|
Number of Sections in the JEE Advanced sample paper PDF download |
Three Sections
|
|
Total Number of Questions and Marks |
|
|
Types of Questions |
Four Types
|
|
Marking Scheme |
MRQ
MCQ
NUM
MATCH
|
|
Availability of JEE Advanced sample papers free download PDF |
At www.selfstudys.com |
JEE Advanced Year-Wise Sample Papers
You can explore the JEE Advanced sample papers of several years on our website. The year-wise format gives a huge range of practice questions. You will be able to develop different exam-taking strategies as well with the help of these sample papers.
| Year | Sample Paper |
| JEE Advanced Sample Paper 2024 |
JEE Advanced Sample Paper With Solutions
To make your preparation very effective, you can practice the JEE Advanced sample papers with solutions PDF free of cost on our platform. In each paper, you will get solutions with detailed explanations for all the questions. You can cross-check your responses after solving the questions and rectify any kind of mistakes. These solutions are prepared by the highly expert team of SelfStudys with accuracy.
JEE Advanced Entrance Exam Sample Paper In Subject-Wise Format
The JEE Advanced exam is famous for its challenging nature. To truly excel, you need to go beyond textbooks and go through the practice problems that mirror the actual exam. The JEE Advanced Entrance Exam sample papers are an invaluable resource for familiarizing yourself with the question format of each subject.
In this section, we provide a subject-wise breakdown of what to expect from JEE Advanced sample paper PDF download.
JEE Advanced Sample Paper For Maths
- Examines: Algebra, Calculus, Coordinate Geometry, Vectors, 3D Geometry, Probability, and Statistics.
- Sample papers typically include A mix of short-answer and long-answer questions in the Maths JEE Advanced sample papers free download PDF. They test your problem-solving skills, analytical ability, and application of mathematical concepts.
- Focus on: Mastering important formulas, practicing different approaches to problems, and developing time management skills to tackle lengthy questions.
Physics JEE Advanced Sample Paper
- Examines: Mechanics, Electricity and Magnetism, Optics, Waves, Heat and Thermodynamics, Modern Physics.
- Sample papers typically include A mix of conceptual and numerical problems. You can expect questions testing your understanding of fundamental laws, and ability to apply formulas and solve for unknowns in the JEE Advanced sample papers.
- Focus on: Identifying the core concepts being tested, analyzing diagrams, and applying relevant formulas efficiently.
JEE Advanced Sample Paper For Chemistry
- Examines: Physical Chemistry, Inorganic Chemistry, and Organic Chemistry.
- JEE Advanced sample papers typically include Questions testing your knowledge of factual data, ability to interpret graphs, solve for reaction products, and predict trends.
- Focus on: Brushing up on important reactions, chemical equations, and their mechanisms. Practice balancing equations and recognizing patterns in periodic properties.
Eligibility Criteria To Appear In JEE Advanced
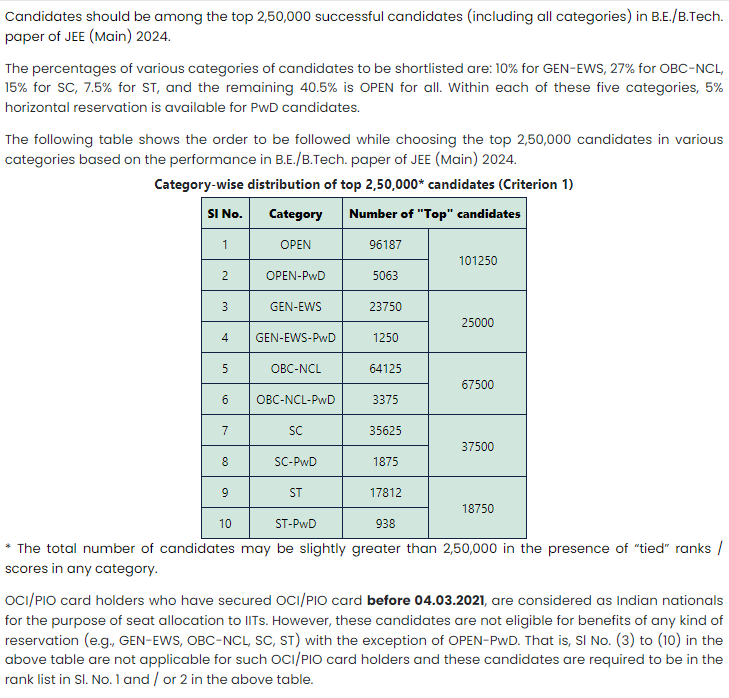
You need to be among the top 2,50,000 successful candidates in the paper of JEE Main in order to take the JEE Advanced examination. For more information on this topic, please go through the image below.
How To Download The JEE Advanced Sample Paper From SelfStudys?
You are allowed to download the JEE Advanced sample papers with solutions PDF from our platform for free. The steps you need to complete in this procedure are as follows:
- The first step is to visit our website which can be done by typing www.selfstudys.com on your internet browser.

- Once the homepage is displayed on your screen, you have to select the “JEE” section from the top menu.

- Now, you can click on the “JEE Sample Papers” option from all the other study materials.
- Then, a new page will open on your browser wherein you need to tap on the “JEE Advanced” icon.
- At last, you can select the JEE Advanced Entrance Exam sample papers to open a variety of papers in it.
- You may also click on the JEE Advanced sample paper PDF download button to get it saved on your device for later practice.
Topics From Which JEE Advanced Sample Paper Is Created
The subject-matter experts of SelfStudys create the JEE Advanced sample papers according to the official syllabus prescribed for this exam. You can have a look at the table below for all the topics from which the questions are asked in this paper.
|
JEE ADVANCED SAMPLE PAPER SYLLABUS |
|
|
Physics |
|
|
General |
General units and dimensions, dimensional analysis; least count, significant figures; Methods of measurement and error analysis for physical quantities of the following experiments: Experiments based on Vernier calipers and screw gauge (micrometer), Determination of g using simple pendulum, Young’s modulus - elasticity of the material Surface tension of water by capillary rise and effect of detergents. Specific heat of liquid using a calorimeter, focal length of a concave mirror and a convex lens using the u-v method, Speed of sound using resonance column, Verification of Ohm’s law using a voltmeter and ammeter, and specific resistance of the material of a wire using meter bridge and post office box. |
|
Mechanics |
Kinematics in one and two dimensions (Cartesian coordinates only), projectiles; Uniform circular motion; Relative velocity. Newton’s laws of motion; Inertial and uniformly accelerated frames of reference; Static and dynamic friction; Kinetic and potential energy; Work and power; Conservation of linear momentum and mechanical energy. Systems of particles; Centre of mass and its motion; Impulse; Elastic and inelastic collisions. Rigid body, moment of inertia, parallel and perpendicular axes theorems; Angular momentum; Torque; Rolling without slipping of rings, cylinders, and spheres; Equilibrium of rigid bodies. Linear and angular simple harmonic motions. Hooke’s Law, Young’s modulus. Law of gravitation; Gravitational potential and field; Acceleration due to gravity; Kepler’s law, Geostationary orbits, Motion of planets and satellites in circular orbits; Escape velocity. Pressure in a fluid; Pascal’s law; Buoyancy; Stoke’s law; Terminal velocity; Bernoulli’s theorem and its applications. Wave motion (plane waves only), longitudinal and transverse waves, superposition of waves, Progressive and stationary waves; Vibration of strings and air columns; Resonance; Beats; Speed of sound in gases; and Doppler effect (in sound). |
|
Thermal Physics |
Thermal expansion of solids, liquids, and gases; Calorimetry, latent heat; Heat conduction in one dimension; Elementary concepts of convection and radiation; Newton’s law of cooling; Ideal gas laws; Specific heats; Isothermal and adiabatic processes, bulk modulus of gases; Equivalence of heat and work; First law of thermodynamics and its applications (only for ideal gases); Second law of thermodynamics; reversible and irreversible processes, Carnot engine and its efficiency; Blackbody radiation: absorptive and emissive powers; Kirchhoff’s law; Wien’s displacement law, Stefan’s law. |
|
Electricity and Magnetism |
Coulomb’s law; Electric field and potential; Electric potential energy of a system of point charges and of electrical dipoles in a uniform electrostatic field; Electric field lines; Flux of an electric field; Gauss’s law and its application in simple cases. Capacitance; Parallel plate capacitor with and without dielectrics; Capacitors in series and parallel; Energy stored in a capacitor. Electric current; Ohm’s law; Series and parallel arrangements of resistances and cells; Kirchhoff’s law and simple applications. Biot-Savart’s law and Ampere’s law; Magnetic field near a current-carrying straight wire. The magnetic moment of a current loop; Effect of a uniform magnetic field on a current loop; Moving coil galvanometer, voltmeter, ammeter, and their conversions. Electromagnetic induction: Faraday’s law, Lenz’s law; Self and mutual inductance; RC, LR, LC, and LCR (in series) circuits with d.c. and a.c. sources. |
|
Electromagnetic Waves |
Electromagnetic waves and their characteristics. Electromagnetic spectrum (radio waves, microwaves, infrared, visible, ultraviolet, x-rays, gamma rays) including elementary facts about their uses. |
|
Optics |
Rectilinear propagation of light; Reflection and refraction at plane and spherical surfaces; Total internal reflection; Deviation and dispersion of light by a prism; Thin lenses; Combinations of mirrors and thin lenses; Magnification. Wave nature of light; Huygens principle, interference limited to Young’s double slit experiment. Diffraction due to a single slit. Polarization of light, plane polarized light; Brewster’s law, Polaroids. |
|
Modern Physics |
Atomic nucleus; Law of radioactive decay; Decay constant; Half-life and mean life; Binding energy and its calculation; Fission and fusion processes; Energy calculation in these processes. Photoelectric effect; Bohr’s theory of hydrogen-like atoms; Characteristic and continuous X-rays, Moseley’s law; de Broglie wavelength of matter waves. |
|
Chemistry |
|
|
General Topics |
Concept of atoms and molecules; Dalton’s atomic theory; Mole concept; Chemical formulae; Balanced chemical equations; Calculations (based on mole concept and stoichiometry) involving common oxidation-reduction, neutralization, and displacement reactions; Concentration in terms of mole fraction, molarity, molality, and normality. |
|
States of Matter: Gases and Liquids |
Gas laws and ideal gas equation, absolute scale of temperature; Deviation form ideality; van der Waals equation; Kinetic theory of gases, average, root mean square and most probable velocities and their relation with temperature; Law of partial pressures; Diffusion of gases. Intermolecular interactions: types, distance dependence, and their effects on properties; Liquids: vapor pressure, surface tension, and viscosity. |
|
Atomic Structure |
Bohr model, the spectrum of hydrogen atom; Wave-particle duality; de Broglie hypothesis; Uncertainty principle; Qualitative quantum mechanical picture of the hydrogen atom: Energies, quantum numbers, wave function, and probability density (plots only), shapes of s, p, and d orbitals; Aufbau principle; Pauli’s exclusion principle and Hund’s rule. |
|
Chemical Bonding and Molecular Structure |
Orbital overlap and covalent bond; Hybridization involving s, p, and d orbitals only; Molecular orbital energy diagrams for homonuclear diatomic species; Hydrogen bond; Polarity in molecules, dipole moment; VSEPR model and shapes of molecules (linear, angular, triangular, square planar, pyramidal, square pyramidal, trigonal bipyramidal, tetrahedral, and octahedral). |
|
Chemical Thermodynamics |
Intensive and extensive properties, state functions, First law of thermodynamics; Internal energy, work (pressure-volume only) and heat; Enthalpy, heat capacity, standard state, Hess’s law; Enthalpy of reaction, fusion, and vaporization, and lattice enthalpy; Second law of thermodynamics; Entropy; Gibbs energy; Criteria of equilibrium and spontaneity. |
|
Chemical and sonic Equilibrium |
Law of mass action; Equilibrium constant and reaction quotient; Le Chatelier’s principle; Solubility product and its applications, common ion effect, pH and buffer solutions; Acids and bases (Bronsted and Lewis concepts); Hydrolysis of salts. |
|
Electrochemistry |
Electrochemical cells and cell reactions; Standard electrode potentials; Electrochemical work, Nernst equation; Electrochemical series, emf of galvanic cells; Faraday’s laws of electrolysis; Electrolytic conductance, specific, equivalent, and molar conductivity, Kohlrausch’s law; Batteries: Primary and secondary, fuel cells; Corrosion. |
|
Chemical Kinetics |
Rates of chemical reactions; Order and molecularity of reactions; Rate law, rate constant, half-life; Differential and integrated rate expressions for zero and first order reactions; Temperature dependence of rate constant. Catalysis: Homogeneous and heterogeneous, activity and selectivity of solid catalysts, enzyme catalysis and its mechanism. |
|
Solid State |
Classification of solids, crystalline state, seven crystal systems, close-packed structure of solids (cubic and hexagonal), packing in fcc, bcc, and hcp lattices; Nearest neighbors, ionic radii and radius ratio, point defects. |
|
Solutions |
Henry’s law, Raoult’s law; Ideal solutions; Colligative properties” lowering of vapor pressure, the elevation of boiling point, depression of freezing point, and osmotic pressure; Van’t Hoff factor. |
|
Surface Chemistry |
Elementary concepts of adsorption: Physisorption and Chemisorption, Freundlich adsorption isotherm; Colloids: types, methods of preparation, and general properties; Elementary ideas of emulsions, surfactants, and micelles (only definitions and examples). |
|
Classification of Elements and Periodicity in Properties |
Modern periodic law and the present form of the periodic table; electronic configuration of elements; periodic trends in atomic radius, ionic radius, ionization enthalpy, electron gain enthalpy, valence, oxidation states, electronegativity, and chemical reactivity. |
|
Coordination Compounds |
Werner’s theory; Nomenclature, cis-trans and ionization isomerism, hybridization and geometrics of mononuclear coordination compounds; Bonding; Magnetic properties and color of 3-d series coordination compounds; Ligands and spectrochemical series; Stability; Importance and applications; Metal carbonyls. |
|
Isolation of Metals |
Metal ores and their concentration; extraction of crude metal from concentrated ores; thermodynamic and electrochemical principles of metallurgy; cyanide process; refining. |
|
Environmental Chemistry |
Atmospheric pollution; water pollution; soil pollution; industrial waste; strategies to control environmental pollution; green chemistry. |
|
Basic Principles of Organic Chemistry |
Hybridization of carbon; 𝝈 and 𝝅 bonds; Shapes of simple organic molecules; aromaticity; Structural and geometrical isomerism; Stereoisomers and stereochemical relationship of compounds containing only up to two asymmetric centers. |
|
Chemistry in Everyday Life |
Drug-target interaction; Therapeutic action, and examples (excluding structures) of antacids, antihistamines, tranquilizers, analgesics, antimicrobials, and antifertility drugs; Artificial sweeteners (names only); soaps, detergents, and cleansing action. |
|
Maths |
|
|
Sets, Relations, and Functions |
Sets and their representations, different kinds of sets, algebra of sets, intersection, complement, difference and symmetric difference of sets and their algebraic properties, De-Morgan’s law of union, intersection, difference, and practical problems based on them. Cartesian product of finite sets, ordered pair, relations, domain and codomain of relations, equivalence relation. Function as a special case of relation, functions as mapping, domain, codomain, range of functions, invertible functions, even and odd functions, onto and one-to-one functions, special functions, sum, difference, product, and composition of functions. |
|
Algebra |
Algebra of complex numbers, addition, multiplication, conjugation, polar representation, properties of modulus and principal argument, triangle inequality, cube roots of unity, geometric interpretations. Statement of the fundamental theorem of algebra, Quadratic equations with real coefficients, symmetric functions of roots. Arithmetic and geometric progressions, infinite geometric series; the sum of the first n natural numbers. |
|
Matrices |
Matrices as a rectangular array of real numbers, equality of matrices, addition, multiplication by a scalar and product of matrices, transpose of a matrix, elementary row, and column transformations, determinant of a square matrix of order up to three, adjoint of a matrix, properties of these matrix operations, diagonal, symmetric, and skew-symmetric matrices and their properties. |
|
Probability and Statistics |
Random experiment, sample space, different types of events (impossible, simple, compound), addition and multiplication rules of probability, conditional probability, independence of events, total probability, Bayes Theorem, computation of probability of events using permutations and combinations. The measure of central tendency and dispersion, mean, median, mode, mean deviation, standard deviation, and variance of grouped and ungrouped data, analysis of the frequency distribution with the same mean but different variance, random variable, mean, and variance of the random variable. |
|
Trigonometry |
Trigonometric functions, their periodicity, and graphs, addition and subtraction formulae, formulae involving multiple and sub-multiple angles, general solution of trigonometric equations. Inverse trigonometric functions and their elementary properties. |
|
Analytical Geometry |
Two dimensions: Cartesian coordinates, the distance between two points, section formulae, and the shift of origin. Equation of a straight line in various forms, the angle between two lines, distance of a point from a line; Lines through the point of intersection of two given lines, equation of the bisector of the angle between two lines, concurrency of lines; Centroid, orthocentre, incentre, and circumcentre of a triangle. Equation of a circle in various forms, equations of tangent, normal, and chord. Parametric equations of a circle, intersection of a circle with a straight line or a circle, equation of a circle through the points of intersection of two circles, and those of a circle and a straight line. Equations of a parabola, ellipse, and hyperbola in standard form, their foci, directrices and eccentricity, parametric equations, equations of tangent and normal. Locus problems. Three dimensions: Distance between two points, direction cosines, and direction ratios, equation of a straight line in space, skew lines, the shortest distance between two lines, equation of a plane, the distance of a point from a plane, the angle between two lines, the angle between two planes, the angle between a line and a plane, coplanar lines. |
|
Differential Calculus |
Limit of a function at a real number, continuity of a function, limit, and continuity of the sum, difference, product, and quotient of two functions, L’Hospital rule of evaluation of limits and functions. Continuity of composite functions, intermediate value property of continuous functions. Derivative of a function, derivative of the sum, difference, product, and quotient of two functions, chain rule, derivatives of a polynomial, rational, trigonometric, inverse trigonometric. Tangents and normals, increasing and decreasing functions, Rolle’s theorem and Lagrange’s mean value theorem, geometric interpretation of the two theorems, derivatives up to order two of implicit functions. |
|
Integral Calculus |
Integration as the inverse process of differentiation, indefinite integrals of standard functions, definite integrals as the limit of sums, definite integral and their properties, fundamental theorem of integral calculus. Integration by parts, integration by the methods of substitution and partial functions, and application of definite integrals to the determination of areas bounded by simple curves. Formation of ordinary differential equations, solution of homogeneous differential equations of first order and first degree. |
|
Vectors |
Addition of vectors, scalar multiplication, dot and cross products, scalar and vector triple products, and their geometrical interpretations. |
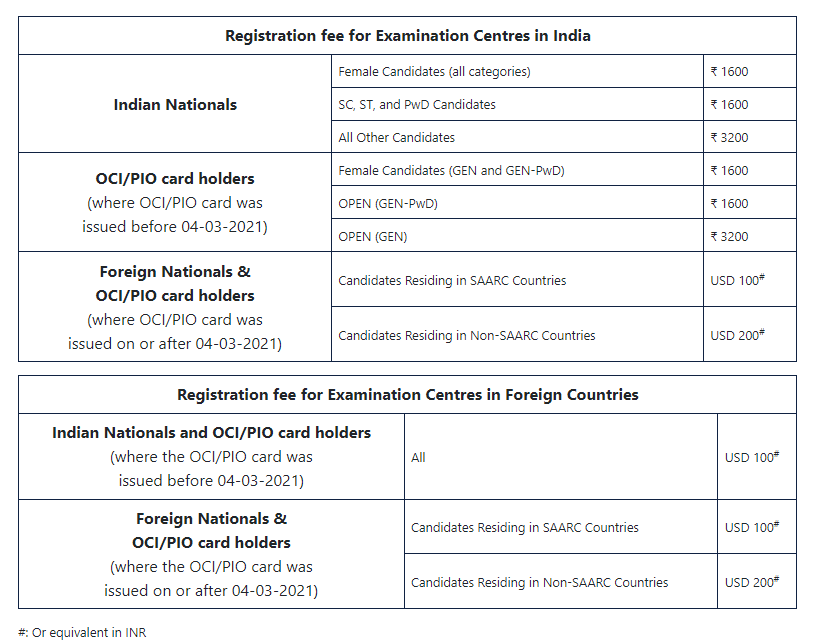
Registration Fee For JEE Advanced
We have gathered the information regarding the registration for the JEE Advanced examination from official sources for your reference. You can check out the image below for fee details.
How To Prepare For Joint Entrance Examination (Advanced) With JEE Advanced Sample Paper?
The JEE Advanced sample papers are a powerful tool to enhance your studies as this examination demands a lot of preparation. A few points to prepare for this exam efficiently with the help of these papers are mentioned below.
Preparation Phase
- Before starting to solve the JEE Advanced sample papers with solutions PDF, you must ensure a solid understanding of the syllabus. Revise your notes, grasp fundamental concepts, and be familiar with important formulas across Physics, Chemistry, and Maths.
- You should find a quiet space free from distractions before solving the paper. Set a timer for 3 hours, mimicking the actual exam duration. You should gather all the necessary stationary - pen, calculator (if allowed), rough sheets - to replicate the exam setting.
Solving The JEE Advanced Sample Paper
- You should spend 5 to 10 minutes going through the entire JEE Advanced Entrance Exam sample papers. Identify the number of questions, the weightage assigned to each section, and the variety of question types (multiple choice, integer type, etc.). This helps strategize your approach.
- You may start by tackling the sections or questions that you feel most confident about. This will build momentum, boost morale, and give you valuable time for tougher problems later.
- Don’t get stuck on a single question in the JEE Advanced sample paper PDF download. Allocate a specific time for each section based on its weightage and your strengths. You should mark questions you are unsure about for later revisiting but don’t spend excessive time on them initially.
- You must read the questions carefully and identify the core of each question. Don’t make assumptions and base your approach solely on the first glace of the JEE Advanced sample papers free download PDF.
- Even for non-multiple-choice questions, you should write down your thought process and calculations clearly. This helps you earn partial marks even if the final answer is incorrect.
- After completing the JEE Advanced sample papers, you should dedicate at least 30 minutes to reviewing your marked questions and attempted solutions. You can utilize the answer key or detailed solutions as well provided with the sample paper.
Analysis Phase
- You must analyze your mistakes in the JEE Advanced sample papers with solutions PDF. Were the conceptual gaps, calculation errors, or time management issues? You should categorize your errors to understand your learning needs.
- You should not just glance through the solutions in JEE Advanced Entrance Exam sample papers. Actively work through them, understand the thought process behind the correct approach, and revisit relevant concepts from your study materials.
- You can make notes or create a separate workbook to record your learnings from the JEE Advanced sample paper PDF download analysis. This personal collection will be a valuable resource for future revision.
- Don’t attempt just one sample paper. You should aim to solve multiple papers from various sources to gain exposure to a wider range of questions and difficulty levels.







 Profile
Profile Signout
Signout










 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










