CBSE Class 10 Science 2024-25: Chapter 3 Metal and Non-metal Competency-Based Questions with Answers; Download Free PDF

SHARING IS CARING
If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
As the CBSE Class 10 board exams get closer, it’s important for students to understand the new exam pattern. Starting in the 2024-25 school year, CBSE will include 50% more competency-based questions. These questions will be both multiple choice and written, focusing on how to use what students have learned in real-life situations.
This article explores Chapter 3: Metal and Non-metal. It highlights key competency-based questions and provides answers to help students succeed. Prepare for your CBSE Class 10 Science exams with important competency-based questions from Chapter 3: Metal and Non-metal.
Understanding Competency-Based Questions in Chapter 3: Metal and Non-metal
Get ready for the CBSE Class 10 Science 2024-25 exams with key competency-based questions from Chapter 3: Metal and Non-metal. These questions help you apply concepts in real-life situations. Download the free PDF for detailed answers and boost your exam preparation.
CBSE Class 10 Science Chapter 3: Metal and Non-metal Important Competency-Based Questions
Answer any four of the following five questions based on the information given below.
Krunal connected a copper plate and an iron plate to the positive and negative terminals of a battery respectively along with a switch. He immersed the plates into a beaker containing acidified copper sulphate solution.
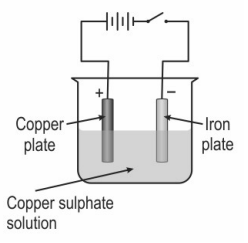
Q: 1 After a few minutes, even before he turned the switch on, he noticed that copper was deposited on the iron plate.
This could have been due to
1. electrolysis
3. a combination reaction
2. electroplating
4. a displacement reaction
Answer. (4)
Q: 2 Which of the following is likely to happen when the current is started?
1. Iron will be deposited on the copper plate.
2. Copper will continue to be deposited on the iron plate.
3. No reaction will occur at the iron plate or at the copper plate.
4. The copper already deposited on the iron plate will go back into the solution.
Answer. (2)
Q: 3 Krunal now replaces the iron plate with a silver plate. He sees that there is no deposition of copper on the silver plate before starting the current. Which of the following could be the reason?
1. Silver is more reactive than iron.
2. Silver is less reactive than copper.
3. Silver is a poorer conductor of electricity than iron.
4. Silver is a better conductor of electricity than copper.
Answer. (2)
Q: 4 What is likely to happen to the concentration of copper sulphate in the solution on passing electric current through the solution in the set-up with the silver plate?
1. It will increase.
2. It will decrease.
3. It will remain the same.
4. (Cannot say without knowing the amount of current passed.)
Answer. (3)
| Download PDF | |
| CBSE Class 10 Science Chapter 1 Chemical Reactions & Equation: Important Competency-Based Questions 2024-25 | Click Here |
| CBSE Class 10 Science Chapter 2 Acids, Bases and Salts: Important Competency-Based Questions 2024-25 | Click Here |
| CBSE Class 10 Science Chapter 3 Metal and Non-metal: Important Competency-Based Questions 2024-25 | Click Here |
| CBSE Class 10 Science Chapter 4 Carbon and its Compounds: Important Competency-Based Questions 2024-25 | Click Here |
Q: 5 Which of the following will happen to the weights of the silver and copper plates after passing the current for some time?
1. The weight of the silver plate will increase and that of the copper plate will decrease.
2. The weight of the copper plate will increase and that of the silver plate will decrease.
3. Both the plates will decrease in weight.
4. Both the plates will increase in weight.
Answer. (1)
Q: 6 Three pieces of a rust free iron rod are completely coated with the following:
(i) plastic
(ii) oil paint
(iii) zinc
An identical scratch is made on each piece, thus exposing the iron. The pieces of iron are kept exposed to moist air for 10 days and then checked for rust formation.
(a) State if rusting will be observed at the point of the scratch on the three iron pieces.
(b) Give reasons for your answer in each case.
(c) Name the process of applying a protective zinc coating to steel or iron.
Answer. (a)
(i) Rust will be seen on the plastic coated iron piece. [0.5 marks]
(ii) Rust will be seen on the painted iron piece. [0.5 marks]
(iii) No rust will be seen on the zinc coated iron piece. [1 mark]
(b)
(i) The iron rod is in contact with air and moisture. [0.5 marks]
(ii) The iron rod is in contact with air and moisture. [0.5 marks]
(iii) Zinc is more reactive than iron and gets oxidised in preference to the iron object. [1 mark]
(c) galvanisation
Q: 7 Listed here is the reactivity of certain metals.
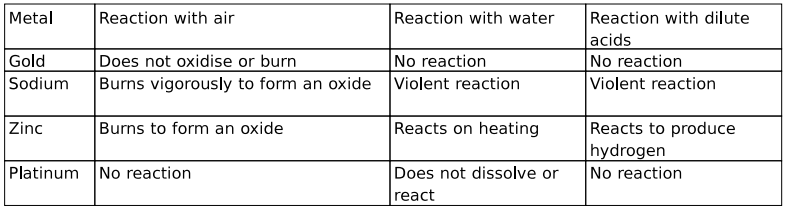
From the list above, identify the metal(s) that are likely to be found in a pure state in the Earth's crust.
Answer. 0.5 marks each for identifying the following:
- gold
- platinum
Q: 8 The blue-coloured solution of the sulphate salt of metal W is taken in a beaker. Metal powders X, Y and Z are added one after the other to the beaker. The colour changes occurring in the solution are shown below.
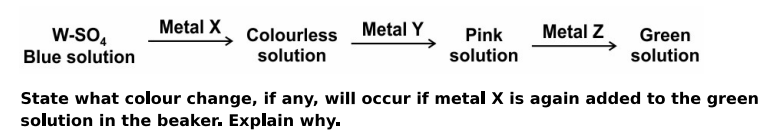
Answer. No colour change will occur.
Metal X is less reactive than metal Z.
OR
Metal X is lower than metal Z in the activity series.
Q: 9 A piece of iron rusts when it comes in contact with air and moisture. Prakash had two identical shiny iron pieces P and Q. To prevent the pieces from rusting, he coated piece P with oil paint and he galvanized piece Q with a coat of zinc metal. He noticed that the coatings were not complete and that a small part of the iron was exposed in both the pieces.
What is Prakash likely to observe about the exposed parts of the two iron pieces after some days? Explain why.
Answer. 1 mark each for the following:
- The exposed part of piece P is rusted.
- The exposed part of piece Q not rusted.
1 mark each for the following:
- Oil painting prevents rusting only by preventing contact of iron with moist air. [1mark]
- Galvanising also protects by zinc getting oxidised in preference to iron as it is more reactive than iron. [1 mark]
Q: 10 Read the following statements.
(P) Stainless steel does not rust.
(Q) Iron, nickel and chromium form an alloy.
Does statement (Q) present a valid explanation for statement (P)? Justify your answer.
Answer. Yes, it does. [1 mark]
Since alloying can change the properties of a metal. [1 mark]
Q: 11 A teacher asks her students to identify a metal, M. She gives them the following clues to help them.
(P) Its oxide reacts with both HCI and NaOH.
(Q) It does not react with hot or cold water but reacts with steam.
(R) It can be extracted by electrolysis of its ore.
(a) Identify the metal.
(b) Write the chemical equations for the reaction of the metal with HCI and NaOH respectively.
(c) What would happen if the metal is reacted with iron oxide?
Answer. (a) Aluminium
(b) 1 mark each for correct equations:
Al2O3 + 2NaOH → 2NaAlO2 + H2O
AI203 + 6HCI → 2AICI3 + 3H₂O
(c) It would displace iron to form aluminium oxide.
Q: 12 A metal oxide on being heated with carbon does NOT produce carbon dioxide.
Give a possible explanation for this behaviour of the metal oxide.
Answer. The metal is more reactive than carbon.
Q: 13 A metallic element, M, has the following properties:
- floats on water
- can be cut with a knife
- occurs naturally as its chloride, of formula MCI
- its oxide dissolves in water to form the hydroxide
(a) State the method of manufacture of the metal M.
(b) Name the major byproduct obtained in the process.
Answer. (a) electrolysis of the molten chloride
(b) chlorine
Q.14 In which of the following forms do electrovalent compounds conduct electricity?
A. only in solid form
B. both in solid form and in aqueous solution
C. both in aqueous solution and in molten form
D. in solid form, molten form and in aqueous solution
Answer. C. both in aqueous solution and in molten form
Q.15
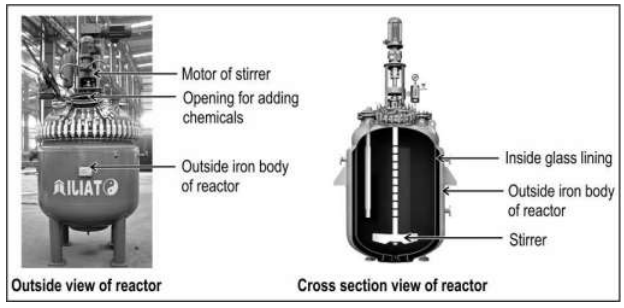
Many chemical reactions like the one shown below, are carried out in glasslined, iron chemical reactors instead of directly in iron reactors

State one advantage of carrying out the reaction in a glass-lined reactor instead of directly in an iron reactor.
Answer. 1 mark for any one of the following:
- no corrosion of the reactor
- no contamination of the product with metal / metal salts
-
👉 Read Also- CBSE Class 10 Half-Yearly/Mid Term 2024-25 : Most Important Questions with Answers; PDF Download (All Subjects)
👉 CBSE Class 10 Study Materials
| CBSE Class 10 Syllabus 2024-25 | NCERT Solutions For Class 10 |
| CBSE Class 10 Previous Year Question Papers | CBSE Class 10 Books |
| CBSE Class 10 Full Study Material | CBSE Class 10 Sample Paper |







 Profile
Profile Signout
Signout












 Quiz
Quiz
 Get latest Exam Updates
Get latest Exam Updates 










